Scientists have found that a protein called phosphorylated α-synuclein, which is related to a few neurodegenerative illnesses, for example, Parkinson’s illness and Lewy body dementia, is likewise engaged with the ordinary cycles of how neurons speak with one another in a sound cerebrum.
The work is distributed in the diary Neuron.
Phosphorylation is a cycle where a phosphate particle is added to a particular amino corrosive, or building block, of a protein; in this situation, the protein is α-synuclein. This expansion can change the state of that protein, making it change its degree of movement.
Most investigations of phosphorylated α-synuclein have concentrated on its role in specific neurological issues, for example, Parkinson’s sickness and Lewy body dementia, where it develops in protein clusters called Lewy bodies. These clusters are believed to be poisonous to neurons, and one of the overarching speculations is that the phosphorylation of the protein α-synuclein triggers these infections.
“Most studies presume that α-synuclein phosphorylation is a marker for pathology in particular illnesses, such as Parkinson’s and Lewy body dementias. Recently, there has been great interest in creating medicines that prevent α-synuclein phosphorylation to treat these illnesses. These findings call into question current assumptions about how these illnesses begin in the brain and may provide insight into how we might treat them more effectively.”
Said Beth-Anne Sieber, Ph.D., program director, NINDS.
“In many examinations to date, the simple presence of α-synuclein phosphorylation is thought to be a marker for pathology for specific problems, similar to Parkinson’s and Lewy body dementias,” said Beth-Anne Sieber, Ph.D., program chief, NINDS. “As of late, there has been impressive interest in creating drugs that forestall α-synuclein phosphorylation as an approach to treating these issues. These discoveries challenge the ongoing speculations about how these issues might start in the cerebrum and may give knowledge into how we could more readily treat them.”
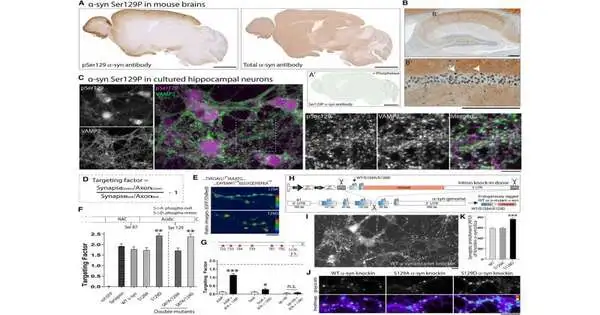
Past work by the lab of Subhojit Roy, M.D., Ph.D., teacher at the College of California, San Diego, and senior creator of the review, proposed that in a sound mind, the α-synuclein protein restrains extreme neuronal terminating to control neuronal correspondence. While investigating this hypothesis, Roy’s gathering startlingly observed that phosphorylation was vital for the typical capability of α-synuclein.
Utilizing a sub-atomic displaying procedure to take a gander at the construction of α-synuclein, Roy and his partners, led by postdoctoral specialist Leonardo Parra-Rivas, Ph.D., found that when α-synuclein is phosphorylated, its construction changes in a way that advances cooperation with different proteins in solid minds.
Besides, they noticed a relationship between expanding brain movement electrically or synthetically and an expansion in how much phosphorylated α-synuclein there is in both refined cells and mouse cerebrum tissue. This finding suggests there may be a connection between synaptic movement and α-synuclein phosphorylation.
Furthermore, tests show phosphorylation is essential for α-synuclein to assume part in gathering an organization of proteins to tie up synaptic vesicles—pockets that discharge synthetic substances empowering neurons to speak with each other and different cells—and to slow neuronal action. Thusly, phosphorylated α-synuclein acts nearly as a brake or grip component to hold action in specific neuronal circuits under tight restraints, recommending that it could play a part in solid minds, which had not recently been explored.
“Looking back, we hadn’t been taking a gander at synuclein phosphorylation the correct way,” said Roy. “Take, for example, the circuits in the olfactory bulb, which, as per our information, have elevated degrees of phosphorylated α-synuclein. The nose smells constantly, so it should be dynamic. One theory is that synuclein phosphorylation might have developed as a security system to safeguard neuronal circuits that should be hyperactive.”
The steady presence of α-synuclein phosphorylation in specific cerebrum districts could mirror a requirement for this biochemical state in those areas. Extra examinations are expected to comprehend how moderately low-recurrence occasions in a solid mind, when collected over a long period, can set off the neurotic gathering of α-synuclein into Lewy bodies, prompting Parkinson’s sickness and Lewy body dementias.
Besides, treatments intended to impede the phosphorylation of α-synuclein itself might have to consider the accidental unfriendly outcomes of hindering a cycle that might be useful to keep neurons practical during peak movement periods.
More information: Leonardo A. Parra-Rivas et al, Serine-129 phosphorylation of α-synuclein is an activity-dependent trigger for physiologic protein-protein interactions and synaptic function, Neuron (2023). DOI: 10.1016/j.neuron.2023.11.020