Physicists and specialists have been attempting to make progressively effective, reasonable, and stable battery innovations to control a large number of electronic gadgets. To do this, they have been utilizing the multi-redox responses of materials that are exceptionally plentiful on the planet, for example, iron and manganese, which could assist with bringing down battery creation costs.
A group of specialists at Seoul Public College has recognized a-LiFeSO4F, an undefined iron fluoride cathode that could be utilized to foster more reasonable high-limit batteries. This terminal, introduced in a paper in Nature Energy, explicitly fills in as a cathode (i.e., the emphatically charged cathode in a battery cell, through which electrons enter electronic gadgets).
The iron fluorisulfate cathode they made was enlivened by a nanocomposite material that they had presented in one of their past works, where they likewise clarified its supporting redox component and surface transformation response. This material is made out of nanosized lithium and a transition metal compound.
“We previously investigated methods for gaining greater capabilities from our nanocomposite cathode material. We were able to gain a better knowledge of the conversion reaction and mechanochemical synthesis because the material’s redox mechanism is akin to a conversion reaction and is normally produced by high intensity ball milling.”
Kisuk Kang, one of the researchers who carried out the study,
“Beforehand, we were reading up on the techniques for getting extra limits from our nanocomposite cathode material,” Kisuk Kang, one of the scientists who did the review, told TechXplore. “As the redox instrument of the material is like a transformation response and is ordinarily integrated by the high-energy ball processing, we had the option to achieve a superior comprehension of the change response and mechanochemical blend.”
The group’s new review depends on the perceptions and discoveries gathered in their past examination. Their previous discoveries prompted them to investigate the possibility that the transformation response of their nanocomposite cathode material, combined with an intercalation ability, would allow them to open the extraordinary limit of the change metal oxides contained within it.
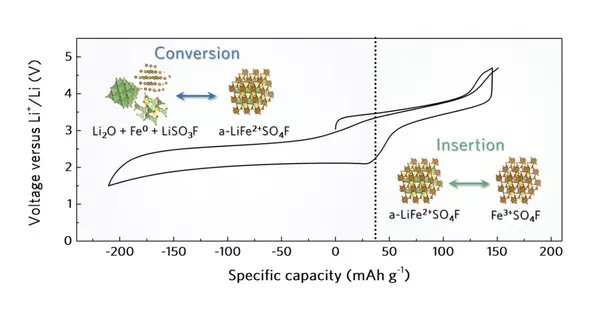
“During the improvement of this idea, we contemplated on the best way to successfully upgrade the reversibility of an intercalation/transformation double-sort terminal material,” Kang made sense of. “We arrived at the resolution that an undefined construction could assist with accomplishing this, taking into account the idea of the transformation response.”
The cathode acknowledged by Kang and his partners is made of LiFeSO4F, yet with an undefined (i.e., not translucent) structure. This material could be effortlessly integrated utilizing mechanochemical processes, explicitly through the high-energy ball processing of lithium fluoride (LiF) and iron sulfate (FeSO4).
A wonderful benefit of the scientists’ cathode is that it upholds the reversible inclusion and extraction of lithium particles through two cycles known as intercalation and change. This fundamentally builds its total limit, which could thus work on a battery’s duration and execution.
“Our review divulged the original job of nebulous design in empowering the reversibility of double-kind intercalation/change terminal material,” Kang said. “The reversible and concurrent use of intercalation and transformation responses in the undefined construction is broadly applicable to other change metal mixtures and thus expands the competitor gathering of high-energy thickness cathode material.”
Later on, the cathode presented by this group of specialists could be utilized to make high-capacity and low-cost battery innovations. Furthermore, its hidden synthetic cycles could be repeated utilizing other progress metal mixtures, opening up new opportunities for the production of highly performing cathodes based on earth-abundant materials.
“Since we tested our idea, we have been investigating a more extensive compositional space to distinguish other conceivable cathode materials,” Kang added. “In addition, we are concentrating on the general oversight rules connected with the blend of nebulous design and the relationships among amorphization and intercalation capacity.”
More information: Jaehoon Heo et al, Amorphous iron fluorosulfate as a high-capacity cathode utilizing combined intercalation and conversion reactions with unexpectedly high reversibility, Nature Energy (2022). DOI: 10.1038/s41560-022-01148-w
Sung-Kyun Jung et al, Lithium-free transition metal monoxides for positive electrodes in lithium-ion batteries, Nature Energy (2017). DOI: 10.1038/nenergy.2016.208
Journal information: Nature Energy