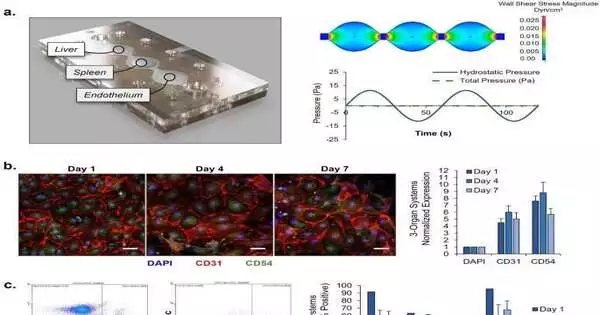
A functional, multi-organ, serum-free system to culture P. falciparum—a protozoan that typically causes severe and fatal malaria—in order to establish innovative platforms for the development of therapeutic drugs was developed by Michael J. Rupar and a team of researchers at Hesperos Inc. in Florida, U.S.
For the purpose of simulating an infection, the platform contained four human organ constructs, including hepatocytes, splenocytes, endothelial cells, and recirculating blood cells. The group utilized two types of P. falciparum: the 3D7 strain, delicate to chloroquine, a deep-rooted enemy of malarial medication, and the W2 strain, safe to chloroquine. They kept up with practical cells in sound and ailing circumstances for 7 days in the recycling microfluidic model.
By significantly reducing parasitemia in the 3D7 strain-constituent model, the researchers demonstrated an effective platform for therapeutic development. They involved this arrangement for restorative record assurance to assess askew poisonousness for those hostile to malarial treatment in a subordinate way. The results can lay out another way to deal with an assessment hostile to malarial treatments in a reasonable human model that kept up with blood dissemination for 7 days.
The existence pattern of Plasmodium falciparum
The parasitic lifecycle happens in two phases: Sporozoites are released by the mosquito from its saliva during a blood meal. These sporozoites then travel through the circulation of the peripheral blood to the liver, where they reproduce within hepatocytes. Within a few days, this results in a large number of merozoites being released from the ruptured hepatocytes and moving from the liver to the bloodstream.
The merozoite lifecycle is asexual and erythrocytic, in which the parasite infects blood cells to grow. Merozoites entered the ring stage within 48 hours thanks to rapid asexual replication fueled by the host’s hemoglobin. Merozoites then matured into trophozoites and schizonts, which continued to grow and replicate until they burst, releasing additional merozoites to continue the infection cycle.
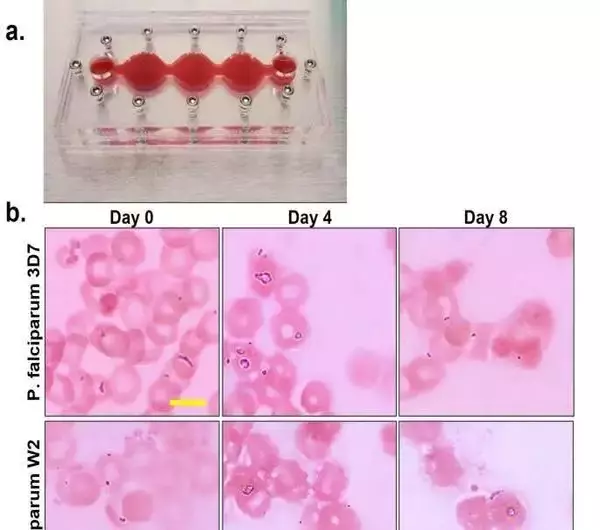
Morphological movement of P. falciparum in the 4-organ jungle fever on-a-chip framework (a) Multi-organ lodging after expansion of 3% essential human red platelets (b) Upon the arrival of framework gathering, contaminated erythrocytes were drawn from constant societies, and every framework was vaccinated at 5-7% parasitemia. Both 3D7 and W2 kinds of P. falciparum multiplied north of an 8-day time span in the frameworks at 2-6%, not set in stone by a meager blood smear and Giemsa stain. Scale bars are 5 m. c) Parasites at all phases of the erythrocytic life cycle were seen following four days of culture in the multi-organ frameworks, including parasites at stage III of gametocyte arrangement. (d) Parasitemia was resolved each 12 h through a flimsy film smear with Giemsa stain. When cocultured in the four-organ systems, the parasitemia of the two strains ranged from 4 to 14%. The mean SEM is represented by data points. Credit: Logical Reports, doi: 10.1038/s41598-023-35694-4
The development of strategies to combat malaria Malaria is a pandemic that is expected to grow from 2014 to 2020. There has been a steady rise in reports that highlight the emergence of resistant strains over time. Subsequently, bioengineers and life researchers are quick to foster new methodologies for hostile malarial advancement to battle sickness.
The Plasmodium parasite causes intestinal sickness and is transmitted by the female Anopheles mosquito. Of the variations, the falciparum species is the deadliest and is essentially liable for an assortment of serious intestinal sickness cases.
The World Wellbeing Association expects to universally decrease jungle fever case rates and mortalities by 90% by 2030. Specialists are quick to accomplish this objective by researching new stages to find solutions for the sickness. A multi-organ, pre-clinical anti-malarial drug discovery platform that can establish and maintain healthy and disease conditions as a cost-effective method for animal models was developed by Rupar and colleagues.
The experimental setup
Rupar et al.’s experimental setup utilized two kinds of P. falciparum: a chloroquine-sensitive and a chloroquine-resistant strain that the multi-organ model successfully maintained for eight days. This instrument was developed by scientists to evaluate malaria treatment in humans, investigate the platform’s effectiveness and safety, and investigate the therapeutic index’s safety for future research. To plate the liver, spleen, and endothelial cells, Rupar and colleagues developed a three-part microfluidic device.
The pumpless framework acquainted the gravity-driven stream with the multi-organ framework with sinusoidal shaking and significant physiological boundaries. They were able to simulate the early stages of a systemic infection with the P. falciparum parasite by engineering the malaria-on-a-chip model without pumps using microfluidics. This allowed them to ensure that primary human red blood cells circulated throughout the model.
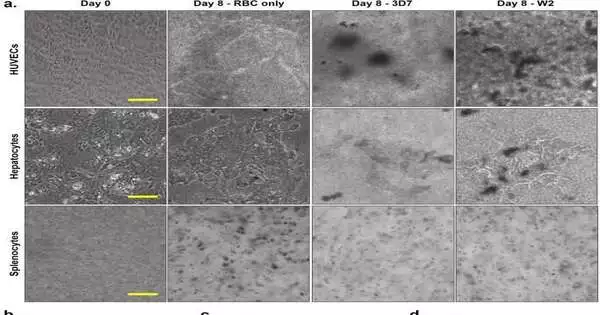
(a) Endothelium, spleen, and liver organs construct morphologies eight days after coculture in four-organ systems with recirculating uninfected or infected RBCs. The concentration of erythrocytes necessary for parasite growth made it difficult to image these organ modules under all conditions. However, the presence of P. falciparum in culture appears to have a significant impact on the morphologies of these cells, as evidenced by apparent morphological differences between organs in uninfected (RBC only) and infected (3D7, W2) systems. (b-d) Feasibility of HUVECs, essential hepatocytes, and essential splenocytes There were no statistically significant differences (n = 12). Credit: Logical Reports, doi: 10.1038/s41598-023-35694-4
Jungle fever on a chip
The researchers portrayed the cells in the multi-organ framework by collecting cells on chips without the parasite on the jungle fever on-a-chip stage. They looked at cell viability, function, and appearance over the course of seven days in a healthy micro physiological system. The team then looked at the parasite’s life cycle inside the instrument using two strains: the 3D7 strain, which is sensitive to chloroquine, and the W2 strain, which is resistant to it.
The analysts saw all stages over the course of 8 days to decide the degree of parasitemia in every framework.
The team then examined the viability of functional cells in the organ-chip instrument, which contained red blood cells that had been infected with either strain or uninfected blood for eight days. Utilizing stage imaging, they caught the utilitarian cells before gathering and following dismantling on day 8. Upon P. falciparum infection, morphological changes indicated that cell viability was disrupted.
Every 12 hours, Rupar and colleagues tracked parasitemia and found significantly lower levels in the treatment group. Albeit this reduction in parasitic levels vacillated between the medication delicate and drug safe frameworks, at last the levels were fundamentally less in the chloroquine delicate treatment bunch when contrasted with the safe frameworks. They continued to investigate the cells’ viability after disassembling the instruments.
Viewpoint
Along these lines, Michael J. Rupar and partners fostered a four-organ intestinal illness model addressing a P. falciparum disease. The team used the model to find out if chloroquine had any off-target effects on the functionality or viability of organ constructs and to develop a therapeutic index for malaria treatment. To demonstrate a cost-effective strategy for studying antimalarial therapy, the serum-free multi-organ construct included a liver, spleen, and endothelium.
This development permits natural chemists and bioengineers to concentrate on parasitic connections continuously inside a microphysiological organ-chip climate and distinguish predictable off-target impacts of the helpful mixtures.
More information: Michael J. Rupar et al, Development of a human malaria-on-a-chip disease model for drug efficacy and off-target toxicity evaluation, Scientific Reports (2023). DOI: 10.1038/s41598-023-35694-4
Louis H. Miller et al, The pathogenic basis of malaria, Nature (2002). DOI: 10.1038/415673a