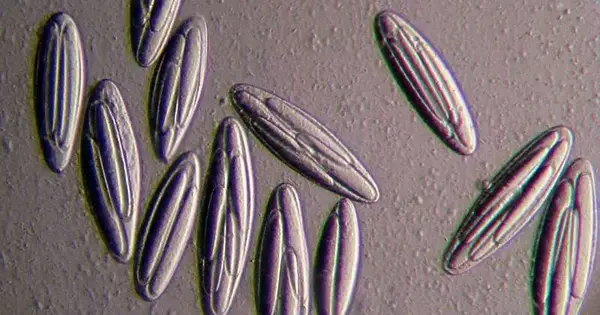
Not all microscopic organisms are equivalent. The majority are tiny, with just a few thousandths of a centimeter of length and one cell. Yet, microbes of the Epulopiscium family are sufficiently huge to be seen with the unaided eye and 1 million times the volume of their better-known cousins, E. coli.
In a review distributed Dec. 18 in Procedures of the Public Foundation of Sciences, specialists from Cornell and Lawrence Berkeley Public Lab have—interestingly—portrayed the full genome of one type of the group of monsters, which they’ve named Epulopiscium viviparus.
“There are so many interesting and one-of-a-kind aspects to this incredible giant bacterium: Esther Angert, a professor of microbiology in the College of Agriculture and Life Sciences and the study’s corresponding author, described the organism’s enormous size, mode of reproduction, and other characteristics. Uncovering the genomic capability of this life form only sort of took our breath away.”
The primary individual from the Epulopiscium family was found in 1985. In tropical marine coral reef environments like the Great Barrier Reef and the Red Sea, all members of the species live symbiotically within the intestinal tracts of certain surgeonfish.
According to Angert, scientists initially considered it to be a specific kind of protozoan due to its enormous size. The name Epulopiscium comes from the Latin roots epulo, signifying “a visitor,” and piscium, “of a fish.” While most microbes imitate by isolating themselves in half to make two posterity, E. viviparus makes upwards of 12 duplicates of themselves, which develop inside a parent cell and afterward get delivered. “Dynamic and swimming—viviparus signifies ‘live birth,'” Angert said.
Concentrating on these monster microbes requires catching the fish in which they live and protecting the cells or extricating DNA and RNA as fast and cautiously as could be expected, said Angert, who for quite a long time has teamed up with fish scientists at Reptile Island Exploration Station in Australia to gather and concentrate on examples.
The specialists were particularly intrigued to figure out how E. viviparus powers its supermetabolic requirements. Microbes that feed off supplements in their current circumstances, as opposed to making their own energy from daylight, for the most part fall into two camps: those who are able to breathe and those who aren’t. Without oxygen, microorganisms frequently use maturation to remove energy, and “aging organic entities simply don’t get as much value for the money from supplements,” Angert said.
Since E. viviparus is, in fact, a fermenter, the puzzle got bigger because of its enormous size, rapid reproduction, and swimming ability—all of which would require more energy rather than less.
The researchers found that E. viviparus has altered its metabolism to make the most of its surroundings. This included using a unique method to generate energy and move (similar to the swimming method used by cholera-causing bacteria) and dedicating a significant portion of its genetic code to the production of enzymes that can extract nutrients from its host’s gut.
The enzymes that are used to make ATP, the energy currency of all cells, are among the most abundantly produced enzymes. According to Angert, the energy-producing and transporting proteins in E. viviparus are housed in a highly folded membrane that runs along the outer edge of the cell. This membrane shares some surprising similarities with the way mitochondria function in the cells of more complex organisms.
“We as a whole know that ‘the mitochondria are the force to be reckoned with of the cell,'” Angert said, “and incredibly, these films in E. viviparus have sort of united on a similar model as the mitochondria: They have a membrane that is very folded, which makes the surface area where these pumps that produce energy can work bigger. This bigger surface area makes a powerhouse of energy.”
This fundamental exploration has a large group of possible future applications, especially as E. viviparus has such powerful methodologies to utilize the supplements found in green growth, Angert said. Green growth is a developing objective for animal welfare, environmentally friendly power, and human nourishment since its development doesn’t rival land-based farming.
The first creator of the review is David Sannino, Ph.D., a previous postdoctoral partner in Angert’s lab. Other co-creators are Francine Arroyo, Ph.D.; what’s more, previous postdoctoral specialists Charles Pepe-Ranney and Wenbo Chen; and, what’s more, Jean-Marie Volland and Nathalie Elisabeth, both with the Lawrence Berkeley Public Research Center.
More information: David R. Sannino et al, The exceptional form and function of the giant bacterium Ca. Epulopiscium viviparus revolves around its sodium motive force, Proceedings of the National Academy of Sciences (2023). DOI: 10.1073/pnas.2306160120