Analysts at the U.S. Branch of Energy’s (DOE) Argonne Public Lab have a long history of cutting edge disclosures with lithium-particle batteries. Large numbers of these disclosures have zeroed in on a battery cathode known as NMC, a nickel-manganese-cobalt oxide. Batteries with this cathode presently power the Chevy Bolt.
Argonne analysts have made one more leap forward with the NMC cathode. The group’s new design for the cathode’s miniature measured particles could prompt longer-enduring and more secure batteries ready to work at high voltage and power vehicles for longer driving reaches. A paper on this exploration showed up in Nature Energy.
“The present-day NMC cathode has represented a significant boundary to activity at high voltage,” said Guiliang Xu, partner scientist. With charge-release cycling, execution quickly declines because of breaks in framing in the cathode particles. For a long time, battery scientists have been looking for ways of killing these bugs.
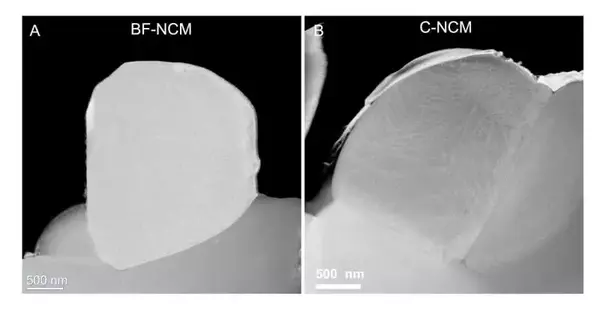
“At the point when we take a gander at the surface morphology of these particles, they seem to be single gems. Yet, when we utilize a method called synchrotron X-beam diffraction microscopy and different procedures at the APS, we find limits concealing inside.”
Physicist Wenjun Liu
One past methodology included microscale round particles comprised of various smaller, more modest particles. The huge round particles are polycrystalline, with diverse glasslike locales. Thus, they have what researchers allude to as grain limits between particles, which cause breaking upon battery cycling. To forestall this, Xu and his Argonne partners had recently developed a defensive polymer covering around every molecule. This covering encompasses the huge round particles and the more modest ones inside them.
An alternate way to deal with keep away from this breaking includes single-gem particles. Electron microscopy of these particles showed they have no limits.
The issue the group confronted was that cathodes produced using both covered polycrystals single gems actually framed breaks with cycling. Thus, they exposed these cathode materials to broad examinations at the High level Photon Source (APS) and Place for Nanoscale Materials (CNM), DOE Office of Science client offices at Argonne.
Different X-beam investigations were done at five APS beamlines (11-BM, 20-BM, 2-ID-D, 11-ID-C, and 34-ID-E). It turned out that what researchers had accepted were single gems, as proven by electron and X-beam microscopy, really had limits inside. Checking and transmission electron microscopies at CNM confirmed the finding.
“At the point when we take a gander at the surface morphology of these particles, they seem to be single gems,” said physicist Wenjun Liu. “Yet, when we utilize a method called synchrotron X-beam diffraction microscopy and different strategies at the APS, we find limits concealed inside.”
Critically, the group fostered a strategy for creating limit free single gems. Testing of little cells with such single-gem cathodes at high voltage showed a 25% expansion in energy capacity per unit volume, with basically no deficiency of execution more than 100 patterns of testing. Conversely, over a similar cycle life, the limit declined by 60% to 88% in NMC cathodes made out of single gems with numerous inner limits or with covered polycrystals.

Limits on inside cathode materials are unwanted on the grounds that they lead to execution debasement.
Estimations at the nuclear scale uncovered the system behind the limit decrease in the cathode. As per nanoscientist Maria Chan in CNM, compared with the locales of them, limits are more helpless towards the deficiency of oxygen iotas when the battery is being charged. This oxygen misfortune prompts debasement with cell cycling.
“Our estimations showed how limits lead to oxygen discharge at high voltage and, hence, execution decline,” Chan said.
Killing the limits forestalls oxygen discharge and, in this way, further develops the cathode security and strength with cycling. Estimations of oxygen discharge at APS and the High Level Light Source at DOE’s Lawrence Berkeley Public Lab upheld this finding.
“We presently have rules that battery makers can use to plan cathode material that is sans limit and works at high voltage,” said Khalil Amine, an Argonne Recognized Individual. “Also, the rules ought to apply to other cathode materials other than NMC.”
More information: Xiang Liu et al, Origin and regulation of oxygen redox instability in high-voltage battery cathodes, Nature Energy (2022). DOI: 10.1038/s41560-022-01036-3
Journal information: Nature Energy