As a flammable fuel, the consumption of hydrogen gas doesn’t contribute to an Earth-wide temperature boost. Today, most hydrogen gas is created from non-renewable energy sources, notwithstanding, and this cycle discharges ozone-depleting substances into the environment. Producing hydrogen gas from clean sources, like the parting of water atoms with power through electrolysis, is critical to accomplishing future carbon nonpartisanship, yet momentum strategies are wasteful and limit the business reasonableness of hydrogen-based advancements.
A new electrocatalyst uses upgraded electrochemical action, response surface region, and solidity to work on the proficiency of hydrogen gas creation by means of electrolysis.
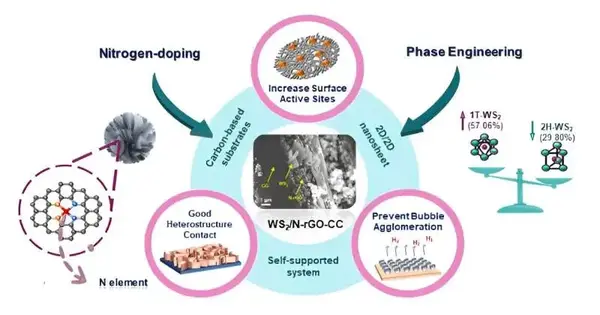
Scientists from Focus of Greatness for NaNo Energy and Catalysis Innovation (Interface) at Xiamen College in Malaysia combined and portrayed a productive and solid water electrocatalyst made out of the progress metal dichalcogenide tungsten disulfide (WS2), a two-layered material with semiconducting properties that has capabilities as an electron acceptor or benefactor in the electrolysis response.
The electrocatalyst, WS2/N-rGO/CC, is made on a carbon material (CC) that will undoubtedly diminish graphene oxide (rGO), a two-layered cross-section semiconductor, joined with a tiny measure of nitrogen (N) to modify the properties of the decreased graphene oxide semiconductor. An aqueous response changes over two-layered WS2 into minute, three-layered bloom-like designs called nanoflowers that increment the surface region of the electrocatalyst to further develop response effectiveness.
“A major problem in clean energy generation is addressed by synthesizing a self-supported electrode for the hydrogen evolution reaction in water hydrolysis. This is an important development. Conventional techniques frequently depend on pricy catalysts and supports, which might reduce the hydrogen production process’s scalability and efficiency. By developing a self-supported electrode that improves electro catalytic activity and provides an affordable and sustainable method of producing hydrogen, our work is a substantial improvement.”
Feng Ming Yap, lead author of the paper and graduate student in the School of Energy.
The group distributed their outcomes in the diary Nano Exploration.
“Blending a self-upheld cathode for the hydrogen development response in water hydrolysis is significant on the grounds that it tends to be a key test in clean energy creation. Customary strategies frequently depend on costly impetuses and upholds, which can restrict the proficiency and versatility of hydrogen creation. Our work addresses a huge progression by making a self-upheld cathode that improves the electrocatalytic action yet additionally offers a savvy and practical answer for the hydrogen age,” said Feng Ming Gab, lead creator of the paper and graduate understudy in the School of Energy and Synthetic Designing at Xiamen College Malaysia in Selangor Darul Ehsan, Malaysia.
Since the dynamic type of electrocatalyst, tungsten disulfide, is straightforwardly integrated into the conductive materials of the cathode, WS2/N-rGO/CC is viewed as a self-upheld terminal. No polymer fasteners or added substances are available in the blended electrocatalyst to cover impetus dynamic destinations or decline electron conductance, amplifying response proficiency.
The exploration group tried different things with consolidating different measures of dimethylformamide (DMF) in the last aqueous combination response to decide the best fixation for the favored metallic 1T stage change of WS2 for the cathode. The terminal created involving a half-grouping of DMF in water (half WGC) during the last aqueous response showed better qualities than cathodes combined utilizing 0, 25, 75, and 100% DMF arrangements.
“Our terminal can effectively deliver hydrogen under an extensive variety of pH conditions, making it flexible and versatile for different down-to-earth applications. It is a stage towards supportable and effective hydrogen creation, which is fundamental for a cleaner energy future,” said Small Jun Ong, boss of the undertaking and academic administrator in the School of Energy and Compound Designing at Xiamen College Malaysia.
Significantly, the half-WGC electrocatalyst outflanked the platinum benchmark electrocatalyst, 20% Pt-C/CC, for the HER in both acidic and fundamental circumstances. In particular, half WGC exhibited a lower overpotential, or energy expected to part water, than 20% Pt-C/CC. The overpotential for half WGC was 21.13 mV, contrasted with 46.03 mV for 20% Pt-C/CC.
The exploration group accepts that more expense and energy-efficient electrocatalysts, as half WGS, are principal to accomplishing the world’s perfect energy objectives. “We intend to investigate the versatility and down-to-earth execution of our self-upheld cathode innovation. Our definitive objective is to add to the change in an economical energy scene where hydrogen can assume a vital role as a spotless and sustainable power source,” said Ong.
Jian Yiing Loh from the School of Energy and Synthetic Designing and the focal point of greatness for NaNo Energy and Catalysis Innovation (Associate) at Xiamen College Malaysia in Selangor Darul Ehsan, Malaysia, were additionally added to the review. This examination is essential for the development of public strategies in Malaysia, specifically the Public Energy Change Guide (NETR) and the Hydrogen Economy and Innovation Guide (HETR), to work with Malaysia’s practical energy in the following five years.
More information: Feng Ming Yap et al, Synergistic integration of self-supported 1T/2H−WS2 and nitrogen-doped rGO on carbon cloth for pH-universal electrocatalytic hydrogen evolution, Nano Research (2023). DOI: 10.1007/s12274-023-6118-8