Dr. Diddo Diddens from the Helmholtz Institute Münster and Prof. Andreas Heuer from the Helmholtz Institute Münster and the Institute of Physical Chemistry of the University of Münster investigated the central question of the extent to which ions in liquid electrolytes move statistically correlated, i.e., in a single direction, in their theoretical work.
With this information, it is possible to determine the individual factors that have an effect on conductivity, such as ion pairs, more accurately. Their study’s in-depth findings have been made public on the pre-print server arXiv and in the Journal of Chemical Physics.
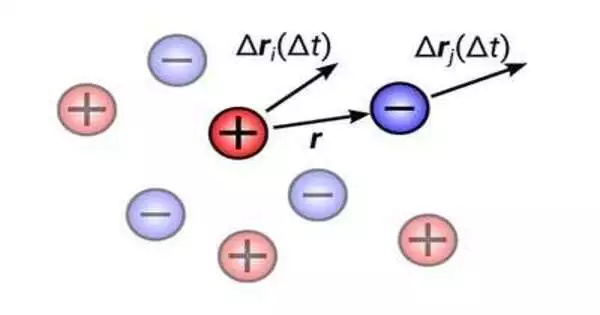
It is commonly believed that mutual repulsion prevents two ions with the same charge from moving in the same direction. The researchers are now in a position to demonstrate that two ions that are adjacent and share the same charge move in the same direction.
“This counter-intuitive behavior can be explained by the fact that, unlike gases, the electrolyte is incompressible as a liquid. As a result, a single ion can only migrate as far as its molecular environment allows.”
Dr. Diddo Diddens from Helmholtz Institute Münster of Forschungszentrum Jülich
“The electrolyte, unlike gases, is incompressible, which makes it possible to explain this counterintuitive behavior. Thusly, a solitary particle can move as its sub-atomic climate permits,” Diddens makes sense of.
On the other hand, the electrostatic interactions—the repulsion of ions with equal charges and the attraction of ions with different charges—primarily affect the ions’ local arrangement but less so the statistically correlated movement of their neighbors.
In the study of batteries, molecular dynamics simulations are frequently utilized in order to ascertain the degree to which specific electrolytes permit the movement of highly correlated individual ions. However, it is frequently challenging to interpret the movements. Diddens and Heuer have now fostered another hypothesis that predicts how much two particles move genuinely together, contingent upon their distance.
The theory makes it possible to better understand how specific factors, like ion pairing, affect conductivity. In liquids, the effects of hydrodynamics are universal. As a result, many electrolytes, including some polymers, contain them.
Understanding how effectively an electrolyte can transport a particular type of ion, like lithium ions in lithium-ion batteries, is aided by figuring out the collective ion dynamics. This helps, among other things, improve the fast-charging behavior of a battery cell. The information can be used to create novel electrolytes.
More information: Diddo Diddens et al, Hydrodynamic Interactions in Ion Transport—Theory and Simulation, arXiv (2023). DOI: 10.48550/arxiv.2302.10330
Diddo Diddens et al, Hydrodynamic interactions in ion transport—Theory and simulation, The Journal of Chemical Physics (2023). DOI: 10.1063/5.0147339