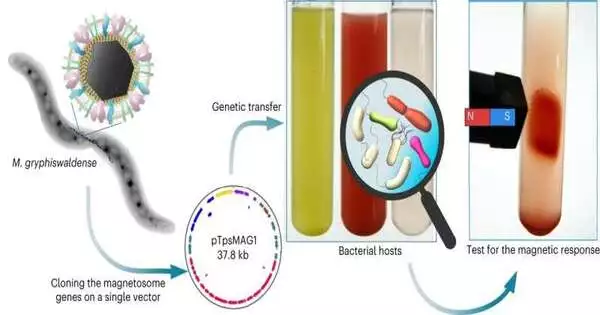
Attractive microscopic organisms have unprecedented abilities due to the attractive nanoparticles, the magnetosomes, which are linked inside their cells. An examination group at the College of Bayreuth has now moved each of the roughly 30 qualities liable for the development of these particles to non-attractive microbes in an expansive series of trials.
This brought about various new bacterial strains that are presently fit for creating magnetosomes. The examination discoveries introduced in Nature Nanotechnology are historic for the age of charged living cells, which have extraordinary potential for the improvement of imaginative, symptomatic, and helpful techniques in biomedicine.
In view of broad examinations, the specialists at first recognized 25 types of non-attractive proteobacteria—by a long shot the most broad space of microscopic organisms—that are especially reasonable for quality exchange and for contemplating magnetosome development. Both biochemical properties and the accessibility of explicit quality groupings were unequivocal variables.
“According to current research, they are well compatible with human cells.” This opens up new possibilities for a wide range of biomedical applications, such as microrobot-controlled transport of active medicinal components, magnetic imaging techniques, and even hyperthermia cancer therapy optimization.”
Dr. Marina Dziuba, who is a research associate at the Microbiology research group in Bayreuth.
Charge was effective in seven species: these microbes persistently produce magnetosomes in which iron-containing magnetite precious stones are tied together in a way like that in the contributor bacterium Magnetospirillum gryphiswaldense.
“As far as future applications in biomedicine, it is especially encouraging that two types of microorganisms that we have effectively hereditarily designed are now generally utilized in biotechnology.”
“As indicated by the present status of the examination, they are well viable with human cells. This opens up new viewpoints for different biomedical applications—for instance, for microrobot-controlled transport of dynamic drug fixings, for attractive imaging strategies, or in any event, for enhancements of hyperthermia disease treatment,” says the main creator of the new review, Dr. Marina Dziuba, who is an exploration partner at the Microbial Science Research Group in Bayreuth.
The Bayreuth specialists have considered the magnetosomes delivered by the new transgenic bacterial strains in additional detail and subsequently distinguished various variables that could be causally associated with magnetosome arrangement.
The correlation between the genomes of these strains and the genomes of those hereditarily changed microorganisms that neglected to create magnetosomes has additionally prompted significant experiences. There is a lot of proof to propose that the magnetosome development of transgenic bacterial strains is firmly connected with their capacity to photosynthesize or to participate in oxygen-free, supposed anaerobic breathing processes.
Generally, the new review shows that it isn’t a single or a couple of specific qualities that transgenic microbes need when they are unequipped for magnetosome development. Rather, the definitive variable for them to integrate magnetosomes subsequent to getting the unfamiliar quality bunches is a blend of specific metabolic properties and the capacity to proficiently utilize the hereditary data of the unfamiliar qualities to create cell proteins.
“Our review shows that further examination is expected to comprehend the biosynthesis of magnetosomes exhaustively, distinguish obstructions to their exchange, and foster procedures to beat them. Simultaneously, in any case, our outcomes shed new light on metabolic cycles that help magnetosome arrangement. They subsequently give structure to future examinations en route to planning new types of biocompatible, attractive microscopic organisms custom-made for biomedical and biotechnological developments,” makes sense of Prof. Dr. Dirk Schüler, Chair of Microbial Science at the College of Bayreuth.
In prior research, the Bayreuth group had proactively prevailed with regards to presenting the qualities liable for magnetosome development from the bacterium Magnetospirillum gryphiswaldense—a model living being for research—into the genome of non-attractive microbes. Notwithstanding, in a couple of cases, this quality exchange brought about hereditarily changed microscopic organisms that, thus, started to shape magnetosomes.
It remained totally muddled as to which elements could impact whether transgenic microorganisms delivered magnetosomes. Against this foundation, the concentrate currently distributed, in which an exploration accomplice at the College of Pannonia in Veszprém/Hungary likewise took part, gives significant new catalyst to the designated charge of living cells.
More information: Dziuba, M.V., Müller, FD., Pósfai, M. et al. Exploring the host range for genetic transfer of magnetic organelle biosynthesis. Nature Nanotechnology (2023). DOI: 10.1038/s41565-023-01500-5 www.nature.com/articles/s41565-023-01500-5