Scientists at the University of Massachusetts Amherst recently reported in the Proceedings of the Public Foundation of Sciences that consistently charged macromolecules—or particles, for example, proteins or DNA, that contain numerous iotas all with a similar electrical charge—can self-assemble into massive designs.This finding overturns how we might interpret how a portion of life’s essential designs are fabricated.
Generally, researchers have perceived charged polymer chains as being made up of more modest, consistently charged units. Such chains, called polyelectrolytes, show unsurprising ways of behaving in self-association in water: They will repulse each other on the grounds that charged objects could do without being near one another. In the event that you add salt to water containing polyelectrolytes, atoms loop up on the grounds that the chains’ electrical shock is screened by the salt.
Nonetheless, “the game is totally different when you have dipoles,” says Murugappan Muthukumar, the Wilmer D. Barrett Teacher in Polymer Science and Designing at UMass Amherst and the review’s senior creator.
While numerous particles have either a positive or negative charge, dipoles have both. This implies that polymers made out of dipoles act uniquely in contrast to the more natural polyelectrolytes, which have either a positive or negative electrical charge: they grow in a pungent arrangement and can shape cross-joins with other dipole polymer chains, which then prompts the development of perplexing polymer structures.
“The discovery that dipoles drive polymer formation has enormous implications. because it sheds new light on one of life’s most fundamental mysteries”
Murugappan Muthukumar, Professor in Polymer Science and Engineering at UMass Amherst
Di Jia, who finished this exploration as a component of her postdoctoral preparation at UMass Amherst and is the review’s lead creator, says that “dipoles can cause polyelectrolytes to act more like polyzwitterions, which show a ‘hostile to polyelectrolyte impact.” This impact is likewise an element of the customary compound polyzwitterions, whose dipoles are made of substance bonds. Hence, for physical polyzwitterion[s] in weakening arrangements, the polymer size increments with expanding ionic strength, showing a globule-to-loop change due to the intra-chain dipole connections. “
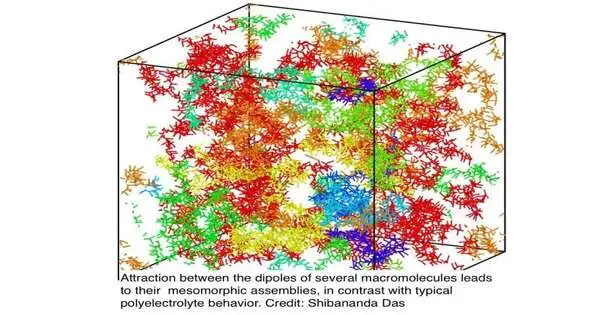
Dipolar polymers are fit for shaping perplexing, automatic designs, which could be utilized in all kinds of things, from drug-conveyance frameworks to cutting edge polymers. “We guess that these dipolar powers in charged macromolecules play a huge part in practically all natural gathering processes, like the unconstrained birth of membraneless organelles,” says Muthukumar.
Besides, these dipole-made polymers show an “in the middle between” state, called “mesomorphism.” In the mesomorphic express, the polymers are neither broadly scattered nor firmly wound, yet congregated into huge, steady, uniform designs that can “self-toxin,” or break up.
“The meaning of the revelation that dipoles drive the gathering of polymers is huge,” says Muthukumar, “on the grounds that it illuminates one of the key secrets of life’s cycles,” or the way in which natural materials know how to self-collect into lucid, stable designs.” “The hypothesis changes the worldview of our opinion on these frameworks, and features the unacknowledged job that dipoles play in the self-gathering of natural materials.”
More information: Di Jia et al, Dipole-driven interlude of mesomorphism in polyelectrolyte solutions, Proceedings of the National Academy of Sciences (2022). DOI: 10.1073/pnas.2204163119
Journal information: Proceedings of the National Academy of Sciences