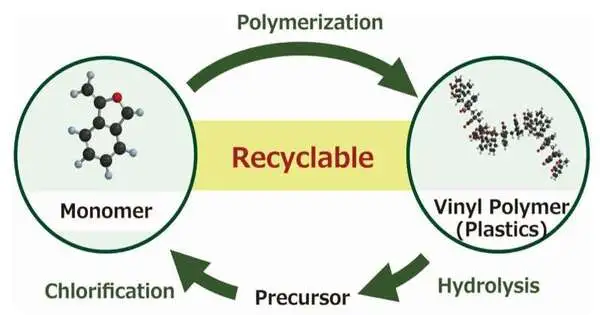
Compound reusing of generally utilized vinyl polymers (VPs) is one of the key innovations expected for understanding a manageable society. In such a manner, a group of specialists from Shinshu College have as of late detailed another synthetic interaction that works with the depolymerization of cyclic styrene-based VPs, bringing about the recuperation of a monomer forerunner.
This exceptionally productive compound reusing framework can assist with successful asset flow and the advancement of new plastic reusing innovations.
Vinyl polymers (VPs) are perhaps one of the most commonly utilized plastic materials. They are found all over, from poly (vinyl chloride) pipes and careful gloves to dispensable polystyrene plates. Given the worldwide need for a move towards supportability, could it not be perfect to synthetically reuse this broadly involved polymer for understanding a feasible society?
As of late, a group of specialists led by academic administrator Yasuhiro Kohsaka from the Staff of Material Science and Innovation (FTST) and Exploration Drive for Supra-Materials (RISM), both at Shinshu College, embraced a review to find an exit plan for accomplishing this.
“Polymers that are stable have low recyclability, whereas those that are easily recyclable are unstable in nature. We resolved this trade-off by abandoning traditional tactics that attempt to reverse the polymerization reaction to recover monomers and devising a two-step recycling approach. In the first step, the polymer was degraded to a monomeric precursor, which was then recovered through chemical modification.”
Associate Professor Yasuhiro Kohsaka from the Faculty of Textile Science and Technology (FTST).
In their new advancement, distributed web-based in ACS Large Scale Letters and co-created by Yota Chiba from the FTST at Shinshu College, the group introduced another technique for successfully depolymerizing the VPs of cyclic styrene subsidiaries to recover a monomer antecedent.
In general, synthetic VP reusing has been tested for a very long time. The ordinary way to deal with reusing any polymer is to switch the polymerization interaction, which involves breaking a solitary, huge particle comprised of rehashing monomeric units down to its parent monomeric parts.
Depolymerizing VPs is troublesome on the grounds that the covalent carbon bonds keeping intact the monomer units are entirely steady and, in this manner, extremely easy to break. Studies have proposed ways of breaking the carbon spine of VPs, yet the vast majority of them neglect to guarantee quantitative and particular scission (bond breaking) of their fundamental chain, which is significant to the viable recuperation of monomers.
“Polymers that are steady have unfortunate recyclability, and the ones that are effectively recyclable are shaky in nature,” says Dr. Kohsaka. “We defeated this compromise by swearing off ordinary procedures that attempt to invert the polymerization response to recuperate monomers and fostering a two-step reusing process. In the initial step, corruption of the polymer to a monomeric forerunner was accomplished, which was followed by the recuperation of the monomer by substance change.”
The group picked VPs made of cyclic α-subbed styrene subsidiaries, for example, 3-methylene phthalide, as their particles for testing synthetic recyclability and examining the ring-opening response of the pendant gatherings within the sight of a base like sodium hydroxide. They tracked down that the launch of the rings because of saponification expanded the steric impediment around the pendant gatherings, which prompted principal chain scission and depolymerization of the VP into monomer forerunners.
These recuperated antecedents were then switched over completely to monomers by means of single-step chlorination and unconstrained intramolecular esterification. The scientists further found that a similar cyclic monomer structure that worked with depolymerization was likewise liable for elevating polymerization, inferable from diminished steric prevention around the vinylidene bunch. These discoveries drove the specialists to infer that cyclic α-subbed styrene subordinates might possibly reuse synthetics.
At a more extensive level, this study has opened new roads for asset dissemination, one of the essential mainstays of a practical society, by giving a simple strategy for polymerization and depolymerization of pervasive VPs. The analysts accept that their discoveries can give helpful grain to additional exploration on the depolymerization of plastic materials as well as the advancement of new recyclable plastics.
“The point of our examination was to help the mission of creating proficient plastic reusing innovation, which is an instrument that mankind frantically needs against the backdrop of natural contamination brought about by plastics. While we can’t eliminate all the plastic that as of now exists on this planet, we can basically utilize plastic assets accessible to us with our new compound reusing system,” finishes up Dr. Kohsaka.
More information: Yota Chiba et al, Chemically Recyclable Vinyl Polymers by Free Radical Polymerization of Cyclic Styrene Derivatives, ACS Macro Letters (2023). DOI: 10.1021/acsmacrolett.3c00573