The synthetic term alkanes, or paraffins, alludes to natural mixtures that comprise single-fortified carbon and hydrogen iotas, like methane, ethane, and propane, and a few different hydrocarbons. In the long term, alkanes have become broadly utilized in natural science because of their novel compound properties and their function in creating synthetic responses.
For many years, scientists have been investigating the possibility of separating alkanes through a cycle known as “non-oxidative dehydrogenation” to achieve important carbon feedstocks and hydrogen fuel. This could finally have important ramifications for the energy area, as it would provide a minimal-expense strategy for making clean fuel for new hydrogen-based energy arrangements.
Sadly, the C-H bonds in alkanes can commonly just be separated at high temperatures, under bright light, or by utilizing stoichiometric oxidants. This ruins the huge scope of reception of this way to deal with making hydrogen fuel.
“In our experiments, we used solar light energy to drive non-oxidative dehydrogenation of alkanes at room temperature, and the introduction of clean photon energy can overcome the thermodynamic limitation, allowing alkane conversion to occur at ambient conditions with higher selectivity and durability.”
Lu Li, Researchers at Jilin University in China
Scientists at Jilin College in China have recently developed a potential system to accomplish the non-oxidative dehydrogenation of alkanes in visible to near infrared light and at room temperature. Their proposed strategy, presented in a paper distributed in Nature Energy, involves the utilization of platinum/titanium dioxide (Pt/dark TiO2) photocatalysts, in which platinum iotas are close yet not completely fortified.
“The underlying objective of our review was to supplant the business TiO2 utilized in my past work with dark TiO2 , to upgrade the light-retention limit of the impetus,” Lu Li said. “In our tests, we used sun-based light energy to drive the non-oxidative dehydrogenation of alkanes at room temperature. The presentation of clean photon energy can beat the thermodynamic limit, causing alkane change to happen at surrounding conditions with higher selectivity and strength.”
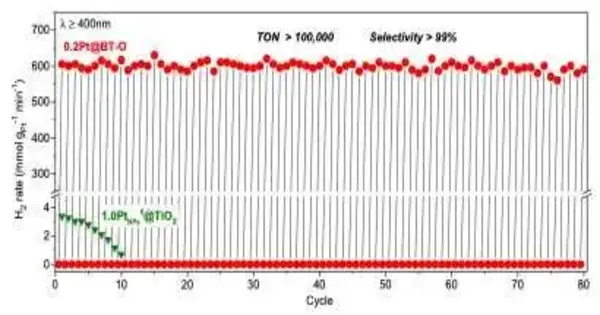
In starting tests, the methodology by Li and his partners accomplished profoundly encouraging outcomes, as it could effectively dehydrogenate various alkanes at room temperature and in apparent to approach infrared light. For cyclohexane, their photocatalysts empowered the creation of H2 with a turnover number of 100,000, with the response really continuous for 80 response cycles. This is an essentially improved outcome than that accomplished by warm responses.
For methane, however, the scientists accomplished a change pace of 8.2%, with a 65% selectivity to propane. Finally, for C2+ alkanes, Li and his partners accomplished a quick dehydrogenation (up to 1,440 mol g1 h1) to the corresponding olefins.
“The pristine’single-iota assortment’ impetuses can join the upsides of single-particle impetuses and nano-impetuses,” Li said. “This new class of impetuses may have likely applications in numerous significant heterogeneous reaction processes.”
Later on, the photocatalysts utilized by this group of analysts could end up being profoundly important for empowering the creation of hydrogen fuel from alkanes without the requirement for high temperatures, UV light, and stoichiometric oxidants. This could assist in bringing down the expense of hydrogen fuel creation, hence possibly working with its utilization for energy applications.
“To expand this work, we are currently searching for effective photocatalysts in view of modest metals,” Li added. “Besides, we will stretch out the substrates to different non-harmful soaked hydrocarbons.”
More information: Lili Zhang et al, Visible-light-driven non-oxidative dehydrogenation of alkanes at ambient conditions, Nature Energy (2022). DOI: 10.1038/s41560-022-01127-1
Lu Li et al, Simple and Efficient System for Combined Solar Energy Harvesting and Reversible Hydrogen Storage, Journal of the American Chemical Society (2015). DOI: 10.1021/jacs.5b03505
Journal information: Journal of the American Chemical Society , Nature Energy