A Florida State University research group has fostered a better approach to making blue light from a class of materials that shows huge potential for optoelectronic gadgets, including sun-based cells, light-radiating diodes (LEDs) and lasers.
The group, led by FSU Professor of Chemistry Biwu Ma, has published another study in Advanced Energy Materials that outlines a basic and viable method for producing an effective and stable blue light from metal halide perovskites.Metal halide perovskites are an emerging class of semiconductor materials characterized by a cubic gem structure that have great light-emanating properties while likewise being simple and cheap to make.
Researchers, including Ma, have previously created highly effective and stable perovskite-based LEDs for green and red light, but producing proficient and stable blue light has proven difficult.Blue light requires a ton of force, and the blue variety’s virtue frequently diminishes over the long haul.
Without an effective and stable blue light, making white light is unthinkable.
“We devised novel ways for producing efficient and steady blue light from perovskites and fabricating high-performance LEDs. Blue, green, and red are required for a full-color display. Green and red are already doing well, but blue is more difficult. Because blue contains more energy, it is more difficult to create a steady blue.”
Professor of Chemistry Biwu Ma
“We grew new systems to accomplish effective and stable blue light from perovskites and create LEDs with better execution,” Ma said. “In the event that you need a full variety show, you want blue, green, and red. As of now, green and red have a decent exhibition as of now, but blue is more earnestly. It’s difficult to make a steady blue since it has higher energy. “
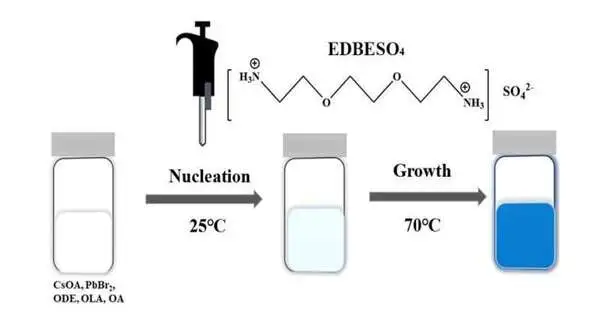
Mama and his group made blue-producing nanoplatelets utilizing a metal halide perovskite in view of the substance compound cesium lead bromide, or CsPbBr3. Nanoplatelets are nanomaterials with a couple of unit cells in thickness and, thus, experience the impacts of quantum control.
To make these specific nanoplatelets radiate proficient and stable blue light, scientists covered them with a multifunctional natural sulfate that’s considered what’s called surface passivation, a profoundly viable strategy used to further develop glow properties and strength.
In this task, the basic natural sulfate passivation helps keep the nanoplatelets from debasing, permitting them to radiate an additional effective and stable blue light.
With these surface passivated CsPbBr3 nanoplatelets as producers, evidence of-idea LEDs were created to show an unadulterated blue light outflow, cresting at 462 nanometers. The luminance of 691 candela for each square meter (the standard unit that determines the splendor of a gadget) and half-lifetime of 20 minutes accomplished in this work are among the best qualities for unadulterated blue perovskite LEDs in view of nanoplatelets answered to date.
“Our work plainly shows the capability of utilizing appropriately surface passivated perovskite nanoplatelets as producers for profoundly effective and stable LEDs,” Ma said.
More information: He Liu et al, Efficient and Stable Blue Light Emitting Diodes Based on CsPbBr 3 Nanoplatelets with Surface Passivation by a Multifunctional Organic Sulfate, Advanced Energy Materials (2022). DOI: 10.1002/aenm.202201605
Journal information: Advanced Energy Materials