The Centers for Disease Control and Prevention (CDC) estimate that more than 2.8 million infections each year are caused by “superbugs” that are resistant to antibiotics and are able to withstand efforts to eradicate them. This situation is a critical threat to public health. Worldwide, researchers are rushing to meet the challenge.
A cooperative group of specialists led by the College of Massachusetts Amherst and including researchers from the biopharmaceutical organization Microbiotix as of late reported that they had effectively figured out how to undermine a critical piece of hardware that microorganisms use to contaminate their host cells and have fostered a test to distinguish the cutting-edge medications to focus on this weak cell hardware and make genuine increases in general wellbeing.
When treating microbial infections, the typical approach is to use an antibiotic to kill the pathogen by entering the harmful cell and killing it. This is not as simple as it sounds because any new antibiotic needs to be oily and water-soluble in order to cross the cellular membrane, the pathogenic cell’s first line of defense, in order to do so. Of course, oil and water don’t mix, so it’s hard to make a drug that has enough of both to be effective.
“I’m a chemist, and I’ve always been fascinated by how chemical molecules interact with living organisms. I’ve been concentrating my research on the chemicals that allow a pathogen to communicate with the host cell it want to infect.”
Alejandro Heuck, associate professor of biochemistry and molecular biology at UMass Amherst.
The difficulty does not end there, as pathogenic cells have developed a device known as an “efflux pump” that can safely excrete antibiotics from the cell where they cannot cause harm. In the event that the anti-toxin can’t conquer the efflux siphon and kill the cell, then the microbe “recollects” what that particular anti-infection resembles and fosters extra efflux siphons to proficiently deal with it—in actuality, becoming impervious to that specific anti-infection.
One way ahead is to see them as another anti-infection, or mixes of them, and attempt to remain one stride in front of the superbugs.
Alejandro Heuck, senior author of the paper and associate professor of biochemistry and molecular biology at UMass Amherst, states, “Or, we can shift our strategy.” I’m a physicist, and I’ve always been extremely keen on understanding how compound particles cooperate with living organic entities. Specifically, I have been zeroing in on the particles that make correspondence conceivable between a microorganism and the host cell it needs to attack.”
Heuck and his colleagues have been particularly interested in the Type 3 secretion system, a communication system that appears to be a pathogenic microbe-specific evolutionary adaptation.
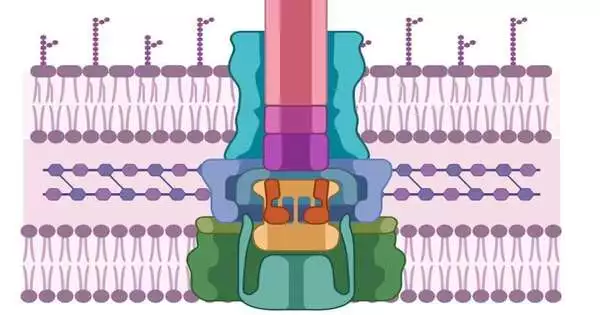
Like the pathogenic cell, cells additionally have thick, challenging-to-enter cell walls. To penetrate them, microorganisms have fostered a needle-like machine that initially secretes two proteins, known as PopD and PopB. Neither PopD nor PopB can break the cell wall independently; however, the two proteins together can make a “translocon,” which could be compared to a passage through the cell film. When the passage is laid out, the pathogenic cell can infuse different proteins that are crafted by contaminating the host.
According to Heuck, “if we don’t try to kill the pathogen, then there’s no chance for it to develop resistance.” This entire procedure is referred to as the Type 3 secretion system, and without PopB and PopD, none of it functions. We are only tampering with its machinery. The disease is still present; it’s simply ineffectual, and it has the opportunity and energy to utilize its regular guards to dispose of the microbe.”
So, how do we find the molecule that can prevent the translocon from coming together?
In some cases, arrangements come to researchers in those “light minutes” when, unexpectedly, all that appears to be legit. It was more of a lightning bug moment in this instance.
Heuck and his partners understood that a protein class called the luciferases—like the ones that make lightning bugs shine around evening time—could be utilized as a tracer. They split the compound into equal parts. One half went into the PopD/PopB proteins, and the other half was designed into a host cell.
Numerous chemical compounds can flood these engineered proteins and hosts. Assuming the host cell out of nowhere illuminates, that implies that PopD/PopB effectively penetrated the cell wall, rejoining the two parts of the luciferase and making them sparkle. Be that as it may, on the off chance that the cells stay dim,? “Then we know which particles break the translocon,” says Heuck.
Heuck is quick to point out that the research done by his team not only has obvious applications for pharmaceuticals and public health, but it also helps us learn exactly how microbes infect healthy cells. He states, “Suddenly we discovered that our findings can help solve a public-health problem.” “We wanted to study how pathogens worked.”
This examination is distributed in the diary of ACS Irresistible Illnesses.
More information: Hanling Guo et al, Cell-Based Assay to Determine Type 3 Secretion System Translocon Assembly in Pseudomonas aeruginosa Using Split Luciferase, ACS Infectious Diseases (2023). DOI: 10.1021/acsinfecdis.3c00482