Strokes cause various changes in quality action in impacted little veins in the cerebrum, and these progressions are possibly targetable with existing or future medications to relieve brain injury or further develop stroke recovery, as per a review driven by Weill Cornell Medicine researchers.
The researchers conducted a comprehensive survey of gene activity changes in small blood vessels in the brain following stroke in a preclinical model for the study, which was published on April 14 in the Proceedings of the National Academy of Sciences. They identified hundreds of genes with significant stroke-driven changes and likely relevance to human strokes by comparing these changes to those observed in stroke patients.
Senior author Dr. Teresa Sanchez, assistant professor of pathology and laboratory medicine and principal investigator of the Laboratory of Molecular and Translational Vascular Research at Weill Cornell Medicine, stated, “Our findings provide a knowledge base that improves our understanding of strokes and points to specific molecules and pathways that can now be investigated as potential targets for future stroke treatments.” Additionally, it is becoming more widely accepted that vascular disease is associated with and contributes to dementia and cognitive dysfunction. This study has recognized sub-atomic elements related to vascular brokenness in the human mind after stroke, a significant reason for dementia.”
“Our results provide a knowledge base that enhances our understanding of strokes and points to specific molecules and pathways that are now able to be investigated as potential targets for stroke treatments,”
Senior author Dr. Teresa Sanchez, assistant professor of pathology and laboratory medicine.
Worldwide, stroke has been the leading cause of death and permanent disability for a long time. Ischemic strokes are the most common type of stroke, caused by a blood clot in a blood vessel that carries blood to the brain. Blood flow is severely restricted or blocked, which reduces oxygen and nutrient delivery to brain cells downstream, resulting in their death or injury and the initiation of inflammatory processes that can cause additional damage.
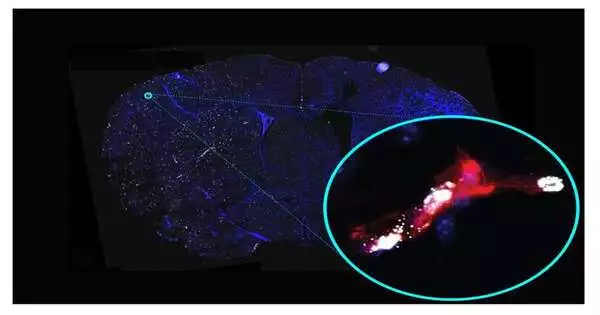
Downstream of the blockage, the “cerebral microvasculature,” or small cerebral blood vessels, are also affected, and their changes are thought to exacerbate post-stroke brain damage. However, because it is technically difficult to record these microvascular changes accurately, they have not been studied as extensively as other aspects of stroke and do not have a specific treatment.
Dr. Sanchez and her team, which includes co-first authors Drs. To overcome these obstacles, Keri Callegari, Sabyasachi Dash, and Hiroki Uchida utilized the most recent optimized methods for studying stroke-affected vessels, which were recently published in Nature Protocols by the Sanchez laboratory. They found alterations in gene activity in the cerebral microvasculature of mice following a stroke that were also observed in studies of human stroke patients.
The researchers discovered 541 genes whose activity was altered in a similar manner in human and mouse cerebral microvessels following stroke. They identified several major clusters by grouping these genes according to their functional roles and connections to diseases. These included clusters related to general inflammation, inflammation in the brain, vascular disease, and the kind of vascular dysfunction that would lead to the leakage of cerebral microvessels. This leakiness implies a weakening of the “blood-brain barrier,” the cellular lining of cerebral microvessels that keeps the majority of the blood’s components out of the brain.
Dr. Sanchez, who is also an assistant professor of neuroscience at the Feil Family Brain and Mind Research Institute, stated, “We found that, following stroke, some molecules that would weaken the blood-brain barrier were upregulated, while others that should protect the blood-brain barrier were downregulated.” Clinical observations of blood-brain barrier disruptions following stroke are consistent with this.”
In addition, the analysis revealed a disruption in the normal activity of genes that regulate sphingolipid levels. These fat-related particles are vigorously engaged with managing veins, and disturbances of their typical activities have been seen in stroke, atherosclerosis, and vascular dementia. When compared to brain tissue, the team discovered that certain types of these sphingolipids are significantly more abundant in cerebral blood vessels. In addition, they found that stroke alters these sphingolipids in the cerebral microvasculature and alters key molecules that regulate their levels. These new findings will make possible the pharmacological targeting of these pathways for the development of stroke therapies.
Many of the molecules whose production had changed following a stroke were evaluated to ensure their “druggability,” or suitability for small-molecule drug targeting. In fact, some of the identified molecules are already the targets of potential drugs to treat other diseases, making it easier to repurpose these drugs for stroke and dementia treatment.
In order to see if this might be beneficial to stroke patients, Dr. Sanchez and her team are now conducting follow-up preclinical experiments with candidate drugs or genetic approaches to reverse some of the specific microvascular changes identified in their study.
She stated, “We’ve generated this knowledge platform and are using it, but we also hope that other scientists will join us in these efforts to develop the first therapies targeting the microvasculature in stroke.” “We’ve generated this knowledge platform and are using it.”
More information: Keri Callegari et al, Molecular profiling of the stroke-induced alterations in the cerebral microvasculature reveals promising therapeutic candidates, Proceedings of the National Academy of Sciences (2023). DOI: 10.1073/pnas.2205786120