College of Maryland specialists concentrated on synaptic changes in a creature model when it created vision, which assumes a critical role in the circadian beat.
In another review highlighted on the front of the journal Scientific Science, named “Microanalytical Mass Spectrometry with Super-Goal Microscopy Uncovers a Proteome Change During Improvement of the Mind’s Circadian Pacemaker,” a group of College of Maryland specialists utilized state-of-the-art advancements to concentrate on a little-figured-out visual pathway in a creature model.
The exploration group set off to comprehend what the improvement of vision means for proteins in a piece of the mind called the suprachiasmatic core (SCN), which is viewed as the vital circadian pacemaker in well-evolved creatures due to its capacity to direct circadian rhythms because of light.
Planned by Peter Nemes, a teacher in the Division of Science and Organic Chemistry, and Colenso Speer, an associate teacher in the Branch of Science, the review originates from a 2019 seed award from UMD’s Cerebrum and Conduct Foundation (BBI) that likewise included Cell Science and Sub-atomic Hereditary Qualities Teacher Najib El-Sayed. Nemes recently got a Beckman Youthful Specialist grant from the Arnold and Mabel Beckman Establishment in 2015, which empowered the development of the mass spectrometer that Nemes further refined at UMD for this most recent undertaking.
Their review intertwined two high-level and different advancements: mass spectrometry and microscopy.
“The BBI grant was so significant for Colenso and me since it permitted us to get together and coordinate these very surprising disciplines,” Nemes said. “I work in science with mass spectrometry, which can distinguish and measure up to 1,000 unique proteins in a neuron. With high-goal microscopy, Colenso can zoom into tissue—down to the degree of single cells, axons, and dendrites—and give wonderful spatial goals.”
With the capacity to pinpoint where neurotransmitters are shaping, the exploration group discovered that different proteins expanded underway when a mouse opened its eyes, interestingly—a significant formative stage connected to circadian mood.
Levels of a protein called synapsin spiked after enlightening because of the development of new neurotransmitters; however, they didn’t come from the retina. All things being equal, they probably started in the nerve center—the district of the mind that houses the circadian pacemaker.
“These discoveries let us know that there are various systems of synaptic association and advancement in particular visual circuits basic for conduct and physiology,” Speer said. “Understanding these distinctions in synaptic explicitness and improvement is a basic move toward understanding the numerous ways that light directs mind capability beyond our thought process of vision.”
Their review is quick to distinguish proteins and picture neurotransmitters in a mouse’s SCN at an early formative stage. While they zeroed in on a particular visual pathway, Nemes and Speer said that specialists have a more extensive interest in understanding the circadian pacemaker, to some degree in light of its significance to human wellbeing.
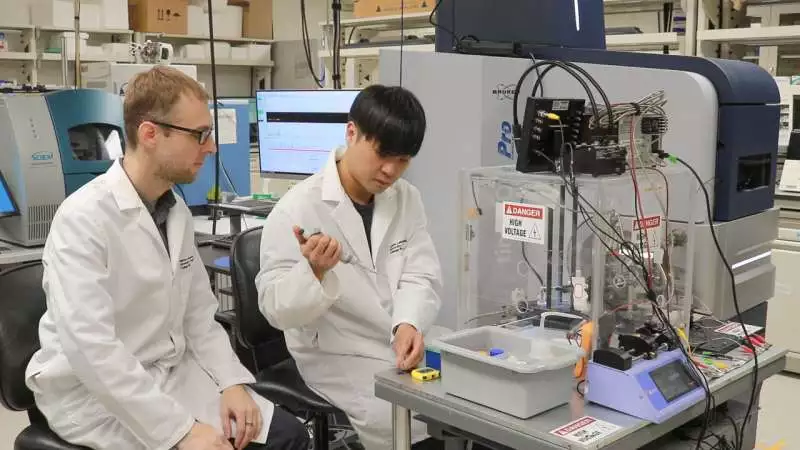
Peter Nemes and Sam Choi set up the proteome of the suprachiasmatic core for mass spectrometry investigation. This exceptionally assembled apparatus can identify many different proteins from restricted tissue and cell microenvironments. Credit: Nathaniel Underland
“There are human wellbeing worries with circadian physiology, most eminently shift work jumble,” Speer said. “A typical one that we as a whole experience the ill effects of eventually is stream slack, which is a distinction between your body’s characteristic circadian mood and the light pattern of the new area that you think of yourself as in.”
Speer noticed that the innovations he and Nemes created could be applied to a large number of studies. Later on, they might reach out to explore human wellbeing and illness.
“We could imagine opening doors for applying this sort of imaging innovation and mass spectrometry to biopsied or posthumous examples as preclinical examination to assist with laying out the shared traits and contrasts between human brain circuits and these creature models that we’re chipping away at in the research facility,” Speer said. “I think this opens up a great deal of energizing open doors as far as understanding how nearby proteins in neural connections are managed.”
Nemes is eager to perceive how their mixed advances will be applied in future examinations, adding that their scientific science paper is only the start.
“By coordinating super-high-responsiveness mass spectrometry with super-goal imaging to find new discoveries, you could apply this to some other setting in the cerebrum,” Nemes said. “This work lays the groundwork for the overwhelming majority of examinations to come.”
More information: Sam B. Choi et al, Microanalytical Mass Spectrometry with Super-Resolution Microscopy Reveals a Proteome Transition During Development of the Brain’s Circadian Pacemaker, Analytical Chemistry (2023). DOI: 10.1021/acs.analchem.3c01987





