Steady lung diseases, ongoing injuries, and medical care-related contaminations are normally considerably harder to treat than different kinds of bacterial diseases. This is on the grounds that they are frequently brought about by biofilms, or at least, states of microorganisms — mostly microbes — that fill up a self-created grid that shields and detaches them from the outer climate.
As of now, another triple-acting anti-toxin specialist has figured out how to get through the biofilm extracellular grid and kill over half of the microorganisms in a single shot, as per a review distributed in the journal npj Biofilms and Microbiomes. The review was driven by Eduard Deluges, teacher at the Branch of Hereditary Quality, Microbial Science and Insights of the Staff of Science and head of the gathering on Bacterial Diseases: Antimicrobial Treatment of the Foundation for Bioengineering of Catalonia (IBEC).
This extracellular grid fuels anti-toxin opposition, one of the greatest dangers to worldwide wellbeing, as per the World Wellbeing Association (WHO), since killing the microbes inside the film depends on multiple times harder. Biofilm diseases are thus the primary ambiguous system of antimicrobial resistance.
Going after these organisms utilizing just anti-toxins isn’t sufficient. There is a requirement for devices that separate the extracellular grid to access and kill the microbes inside. The main creator of this review, which has accomplished this achievement, is Nria Blanco-Cabra, postdoctoral analyst of the gathering driven by Eduard Deluges at the UB and IBEC. The review was done along with researchers from CIDETEC (Basque Country).
A triple-acting medication mix
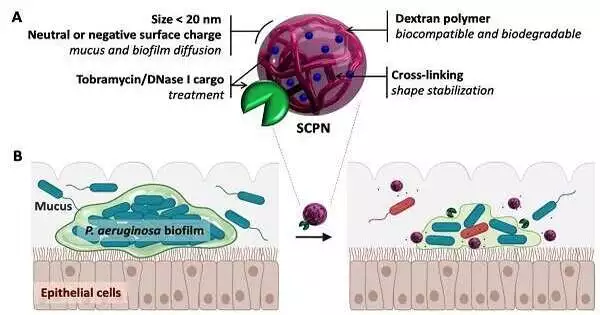
The review zeroed in on the microbe Pseudomonas areuginosa, a microorganism that frequently fills in biofilms in the lungs of those patients with cystic fibrosis or constant obstructive pneumonic illness (COPD), causing steady disease. “We developed biofilm societies in vitro, utilizing a method that looks like the manner in which they exist and fill in nature,” added Deluges.
In clinical practice, these diseases are normally treated with an anti-toxin called tobramycin. Nonetheless, its adequacy is restricted by its failure to enter the film. This happens on the grounds that tobramycin, which is decidedly charged, is killed by the extracellular grid (with a negative charge).
The analysts stacked the anti-toxin into adversely charged nanoparticle transporters. This could kill the positive charge before the medication gets to the biofilm, which would empower it to break the extracellular grid and kill the microbes inside. Furthermore, these transporters, which were powered by dextran-based single-chain nanoparticles, could transport up to 40% of the anti-toxin’s weight.
“Large numbers of the recently concentrated on nanotransporters have just had the option to support a little heap of the objective compound, which has forestalled their clinical use. “We figured out how to beat this snag,” says Deluges.
The anti-toxin stacked nanocarriers were likewise covered in a protein called DNase I. One of the mixtures that holds microbe biofilms together is primary DNA, tracked down all through the extracellular grid. DNase I can separate this “stick,” making the grid relax and permitting the anti-toxin to enter the biofilm much further.
By joining an anti-toxin with several specialists that split the biofilm up, we have made a medication that is more impressive than the anti-toxin alone, which kills the microbes that live inside the film, notes Eduard Deluges, head scientist of the review.
Using microscopy pictures, the analysts found that the new specialist had not just broken up the primary DNA in the extracellular grid, but in addition, that it was following up on and killing the microbes inside. They reduced the bacterial biomass significantly with a single application.
“Having accomplished such huge biofilm leeway with a solitary portion of our representative, we anticipated that a full course of anti-toxins could essentially lessen the weight of these diseases, which are very hard to treat.”
For future clinical purposes, this specialist could be managed in various dosages, as is standard practice with anti-toxins. The following stage is to deal with the clinical approval of this framework. Its commercialization would represent a conclusive development in the treatment of biofilm diseases, the worldwide monetary expense of which right now adds up to 4 billion dollars per year.
More information: Núria Blanco-Cabra et al, Neutralization of ionic interactions by dextran-based single-chain nanoparticles improves tobramycin diffusion into a mature biofilm, npj Biofilms and Microbiomes (2022). DOI: 10.1038/s41522-022-00317-9