Cathode-active materials for lithium-ion batteries are typically created through the mixing and heating of several precursor materials. The specific materials and procedures used can differ based on the required cathode chemistry and battery performance parameters. Layered lithium cobalt oxide, a critical component of lithium-ion batteries, has been manufactured at temperatures as low as 300°C and in as little as 30 minutes.
Lithium-ion batteries (LIB) are the most prevalent form of battery used in consumer devices and electric vehicles. The cathode of LIB for handheld electronics is made of lithium cobalt oxide (LiCoO2). Traditionally, the synthesis of this chemical needs temperatures above 800°C and takes 10 to 20 hours to complete.
A team of researchers from Hokkaido University and Kobe University, lead by Professor Masaki Matsui of Hokkaido University’s Faculty of Science, has devised a new method for synthesizing lithium cobalt oxide at temperatures as low as 300°C and in as little as 30 minutes. Their findings were reported in the journal Inorganic Chemistry.
Lithium cobalt oxide can typically be synthesized in two forms. The high-temperature phase is a layered rocksalt structure, and the low-temperature phase is a spinel-framework structure. Layered LiCoO2 is utilized in Li-ion batteries.
Professor Masaki Matsui
“Lithium cobalt oxide can typically be synthesized in two forms,” Matsui adds. “The high-temperature phase is layered rocksalt structure, and the low-temperature phase is spinel-framework structure. Layered LiCoO2 is utilized in Li-ion batteries.”
Lower-temperature synthesis methods are appealing to the battery industry for a variety of reasons, including better energy efficiency, decreased environmental impact, and the opportunity to create novel types of cathode materials.
The team conducted a series of high-precision experiments under varied conditions to synthesize layered LiCoO2 crystals using cobalt hydroxide and lithium hydroxide as starting materials and sodium or potassium hydroxide as an addition. The procedure was known as the “hydroflux process.” They also discovered the chemical route that resulted in the production of the stacked crystals.
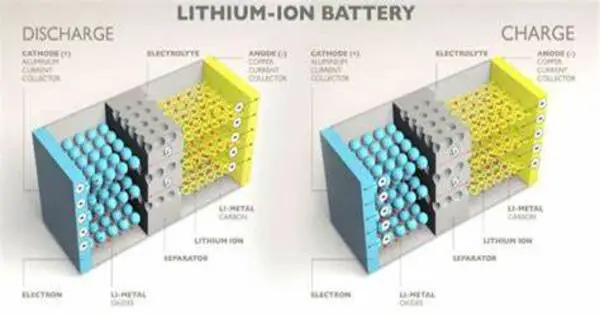
“By understanding the reaction pathway, we were able to identify the factors that promoted the crystal growth of layered LiCoO2,” Matsui said. “Specifically, the presence of water molecules in the starting materials significantly improved crystallinity of the end product.”
The electrochemical properties of the layered LiCoO2 were also examined, and they were only marginally inferior to those of commercially available LiCoO2 created using the standard high-temperature approach.
“This work is the first experimental demonstration of the thermochemical stability of layered LiCoO2 at low temperatures under ambient pressure,” Matsui said in a statement. “The development of this hydroflux process by our team will enable energy-saving measures in a variety of ceramic manufacturing processes.” Based on our understanding of the chemical route, our immediate next steps will seek to improve the hydroflux process.”