Atopic dermatitis, also known as atopy, is a common skin condition that affects dogs. It is caused by an overactive immune response to environmental allergens, such as pollens, molds, and dust mites. Atopy can lead to symptoms such as intense itching, redness, and inflammation of the skin.
There is evidence that atopy may have a genetic component. Studies have identified certain parts of the genome that may be linked to an increased risk of atopic dermatitis in dogs. However, the exact genetic mechanisms underlying this condition are not fully understood, and more research is needed in this area.
If your dog has atopic dermatitis, it is important to work closely with a veterinarian to develop a treatment plan that is tailored to your dog’s specific needs. Treatment may include medications, such as antihistamines or corticosteroids, and lifestyle changes, such as avoiding exposure to allergens and keeping the skin clean and moisturized.
Researchers discovered links between atopic dermatitis (eczema) in dogs and several regions of the genome using new gene mapping methods. Some of the genes discovered are linked to similar problems in humans. For example, the filaggrin gene region, which is thought to be the most powerful risk factor for atopic eczema in humans, has now been linked to the disease in Labrador retrievers.
It’s vital that atopic eczema is correctly diagnosed by careful elimination of other potential non-allergic causes of the patient’s symptoms, followed by a positive allergy test.
Kerstin Bergvall
The findings are published in a new study conducted by the dog genetics group at Uppsala University and the Swedish University of Agricultural Sciences, which has been conducting research in this field for over ten years in collaboration with colleagues in Switzerland, the United Kingdom, and the United States. In the early 2000s, genome sequencing became a reality. Canine genome sequencing has since proven invaluable to researchers attempting to comprehend the human genome.
Dogs have lived alongside humans for tens of thousands of years and suffer from similar diseases, including immunological diseases such as atopic dermatitis (allergic eczema). Studying dog disease genetics, using ordinary blood samples, can also be a way to obtain clues to the causes of the corresponding human diseases.
Dogs and humans affected by atopic eczema have much in common as regards medical symptoms and the early onset of the disease, as well as in histopathological terms with similar immune cell infiltration in the skin. The disease has a complex genetic background in both species and is also influenced by environmental factors.
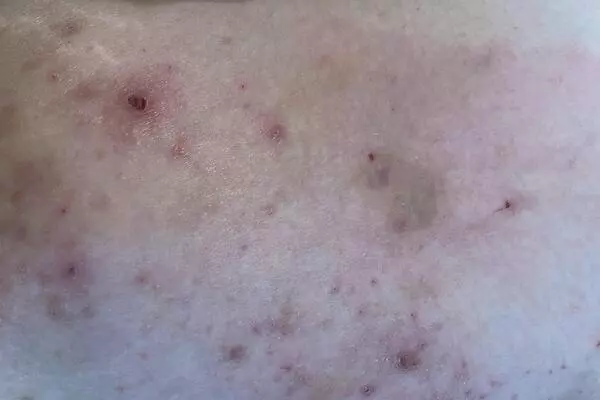
“It’s vital that atopic eczema is correctly diagnosed by careful elimination of other potential non-allergic causes of the patient’s symptoms, followed by a positive allergy test,” says Kerstin Bergvall, veterinarian in charge and specialist in dermatology who has been involved in the research from the start.
With gene mapping technology steadily advancing, new methods have recently emerged for mapping complex diseases. In the study now published in the journal Communications Biology, the researchers used one methodology to capture multiple associated genetic risk variants and another to discover disease variants ‘hidden’ in the genome because of artificially (i.e. humanly) selected characteristics.
“The new methods make it possible to find new risk factors that have become common in the specific breed, perhaps because of the selection for other characteristics,” says Katarina Tengvall, researcher at Uppsala University and first author of the study. “The candidate genes identified here are important for both the nature of the skin barrier and the immune defence, as expected in atopic eczema.”
The study shows several overlaps, or correspondences, with genes associated with human atopic dermatitis. One particularly striking finding now made by the researchers in this study is that the genetic region containing the filaggrin gene, which is regarded as the most powerful genetic risk factor for atopic eczema in humans, is a risk factor in dogs as well.
“This highlights the value of canine studies of genetic diseases that also affect humans. A better understanding of the disease mechanisms may ultimately lead to better therapies for dog and human alike,” says Kerstin Lindblad-Toh, Professor of Comparative Genomics and senior author of the study.





