An on-site and on-demand chemical technology for the environmentally friendly and sustainable use of methane has the potential to emerge from the photocatalytic conversion of methane with water, a ubiquitous and clean oxidant.
Be that as it may, the judicious plan of cutting-edge photocatalysts is ruined by the absence of atomic-level comprehension of opening-driven oxidation energy, dynamic destinations, and resultant photocatalytic execution.
The exploration bunch driven by Toshiki Sugimoto, an academic partner at the Foundation for Sub-atomic Science, has shown that metal cocatalysts stacked on a semiconductor photocatalyst assume basic roles in balancing surface oxidation energy and resultant oxidation selectivity.
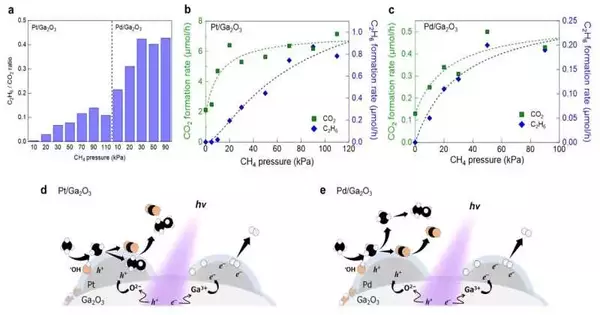
Continuous mass spectrometric investigation of vaporous items under deliberately controlled methane pressures uncovered that the Pt-stacked Ga2O3 photocatalyst transcendently advanced the all-out oxidation of methane toward CO2 on its surface, while the Pd-stacked photocatalyst displayed a higher selectivity for C2H6 development through the gas-stage coupling of free CH3.
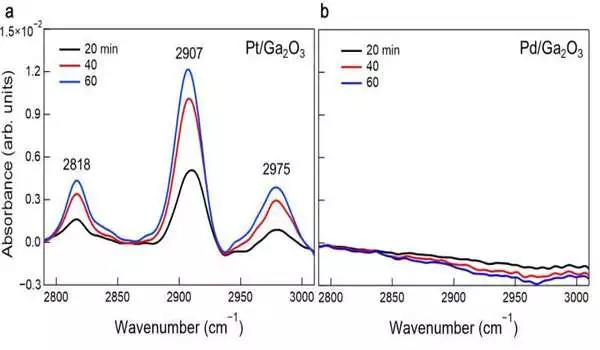
Pt/Ga2O3 and Pd/Ga2O3 photocatalysts’ operando infrared spectra in the C-H stretching region change over time when ultraviolet light is irradiated at methane and water pressures of 30 and 2 kPa, respectively. The presence of the C-H tops on Pt/Ga2O3 exhibits methane oxidation occurring on its surface. No C-H tops on Pd/Ga2O3 show the low population of the hydrocarbon intermediates because of the desorption of the methyl extremist middle. Credit: Toshiki Sugimoto
Operando infrared absorption spectroscopy revealed surface intermediates under working conditions, supporting this difference in methane oxidation kinetics. Besides, the exploration group showed that the Pt catalyst itself was oxidized by photo generated openings.
These exploratory outcomes showed the basic jobs of metal cocatalysts as a supply of photogenerated openings and a compelling response site for methane oxidation processes. For the most part, metal cocatalysts have been recognized as reduction cocatalysts for half a century. These cocatalysts only accumulate electrons from photosynthesis and encourage reduction reactions like H2 evolution.
It is generally accepted that hole-accumulated metal cocatalysts prevent photocatalysis and serve as charge recombination centers. In contrast, this group of researchers confirmed that metal cocatalyst loading accelerated methane oxidation and H2 evolution. These trial results demonstrate that photogenerated electrons and openings are independently caught at various metal cocatalyst particles while staying away from charge recombination and advancing redox responses.
Consequently, the precise operando examination of the photocatalytic oxidation of methane (i.e., the most latent and least complex natural compound) and water (i.e., one of the vital particles in photocatalysis) gives another worldview to the job of metal cocatalysts in photocatalysis and subsequently contributes to fostering a cocatalyst-based surface design methodology for controlling non-warm oxidation responses.
The paper is distributed in the journal Angewandte Chemie Worldwide Release.
More information: Hikaru Saito et al, Beyond Reduction Cocatalysts: Critical Role of Metal Cocatalysts in Photocatalytic Oxidation of Methane with Water**, Angewandte Chemie International Edition (2023). DOI: 10.1002/anie.202306058