Common beliefs about how cancer grows are being challenged by Japanese research led by Kyoto University. In their paper “Transformative accounts of bosom malignant growth and related clones,” distributed in Nature, the exploration group investigates the early transformative occasions prompting disease improvement and the job of non-malignant growth clones that share normal changes.
The scientists utilized phylogenetic investigation to follow the advancement of bosom malignant growth and antecedent sores from procuring introductory driver changes to growing clinically analyzed infections.
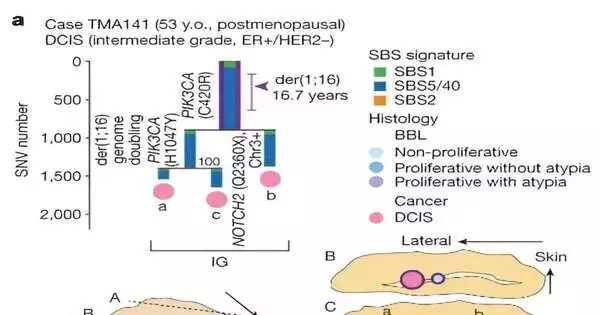
The researchers wanted to learn more about the driver events and their order of occurrence before these clones evolve into cancer, citing recent studies that demonstrate clones with common cancer mutations can exist in healthy tissues. The review zeroed in on bosom diseases holding onto a hereditary modification called der (1; 16), seen in roughly 20% of bosom tumors.
The specialists assessed the surmised timing of early developmental occasions in light of the change rate estimated in ordinary epithelial cells. They discovered that from early puberty to late adolescence, the der(1;16) genetic mutation was acquired. By the patient’s mid-30s, a typical precursor arose, from which both malignant growth and non-disease clones developed.
The scientists performed entire genome sequencing (WGS) of different examples acquired from both disease and clonally related harmless bosom injuries, alongside evidently typical lobules. From WGS, they calculated the rate of mutation accumulation in mammary epithelial-derived single-cell-derived organoids. In view of this transformation rate, they reproduced phylogenetic trees that included both malignant growth and non-disease clones to surmise the whole history of bosom malignant growth advancement.
Using 71 single-cell-derived organoids derived from normal mammary tissues of breast cancer patients and healthy volunteers who were breastfeeding, the researchers estimated the rate of mutation accumulation in normal mammary epithelial cells as they age. The review recognized substantial changes in these organoids and broke down their transformation rates.
A straight relapse model verified that the quantity of single-nucleotide variations (SNVs) essentially relied upon variables like age at test assortment, years after menopause, equality (number of pregnancies arriving at 20 weeks), and the presence of a driver change.
SNVs increased to 19.5 mutations per genome per year prior to menopause but decreased to 8.1 mutations per genome per year after menopause. For every equality occasion, the change number decreased by 54.8, recommending that conceiving an offspring influences the transformation collection rate in mammary cells.
The high decrease of SNVs per equality appears to be considerably larger than specialists anticipated from the ordinary time span of feminine cycle interference by pregnancy (1.1–1.5 years). This could indicate that newly recruited “dormant” stem cells are reconstructing the mammary epithelium. The disappearance of tobacco-signaling clones in the bronchial epithelium following quitting smoking has been proposed to be explained by a similar process.
The number of SNVs increased by 210.4 in the presence of PIK3CA mutations. PIK3CA transformations can make the PI3K compound become overactive, which might make disease cells develop and is related to many kinds of malignant organ growth.
Indels were additionally considered, and their amassing rate was diminished by 45% after menopause, from 1.3 transformations per genome each year to 0.72 changes per genome each year. Every conveyance diminished the transformation number by 3.9.
Intratumor heterogeneity was facilitated by the prevalence of multiple distinct cancer founders from non-cancer ancestors. This is in contrast to the conventional view that the majority of cancers originate in a single cancer founder, indicating that multiple cancer founder cells originating from a population of cells that are not cancerous might be more prevalent than anticipated.
The mutational profile of mammary epithelium is different from that of different tissues and is more impacted by unique changes happening across a lady’s life expectancy, like monthly cycles, pregnancy, conveyance, and breastfeeding. The ebb and flow research indicates the chance of distinguishing precancerous clonal cells well before any disease arises.
More information: Tomomi Nishimura et al, Evolutionary histories of breast cancer and related clones, Nature (2023). DOI: 10.1038/s41586-023-06333-9