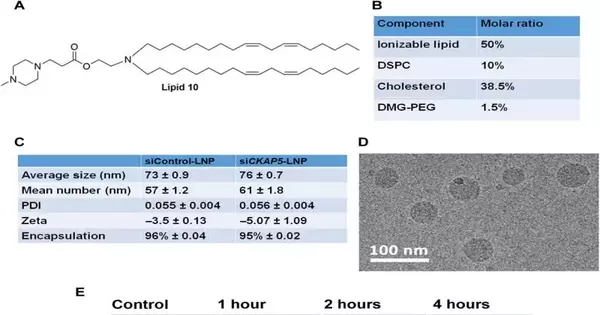
Due to a lack of target-specific agents, the therapeutic potential of microtubule-associated protein targets for cancer therapy remains largely unexplored. At Tel Aviv University in Israel, Sushmita Chatterjee and her colleagues in nanomedicine, materials science, nanotechnology, and biology investigated the therapeutic potential of cytoskeleton-associated protein 5, or CKAP5. Using short interfering RNA (siRNA), a molecular biological method for studying genes, Chatterjee and colleagues silenced the gene by targeting CKAP5 encapsulated in lipid nanoparticles for in vivo delivery. The protein is associated with microtubules.
The specialists screened 20 strong malignant growth cell lines comparative with quality hushing to recognize an exceptionally responsive chemo-safe ovarian disease cell line that went through critical consumption in mitotic shaft elements for viable exploratory disease treatment. The group showed the helpful potential of an ovarian malignant growth model with a 80% endurance pace of quieted CKAP5 lipid nanoparticle-treated creatures. Science Advances is where the study was published.
The results featured the significance of the quality of premium as a remedial objective to research hereditarily temperamental ovarian tumors to additionally clarify its systems of activity.
New treatments for ovarian cancer Ovarian cancer is a common cause of death and is responsible for approximately 4.7 percent of women’s cancer deaths. Neoadjuvant chemotherapy with a drug combination is followed by maximal surgical resection of the tumor as the primary treatment option. About 80%–85% of patients develop chemo-resistance, whereas 60%–80% of patients initially respond to chemotherapy. Consequently, new treatments for ovarian cancer are constantly sought after. Researchers have investigated a few medications focusing on unambiguous receptors, although such endeavors didn’t yield successful outcomes for patients with ovarian malignant growth.
Credit: Science Advances (2023). DOI: 10.1126/sciadv.ade4800
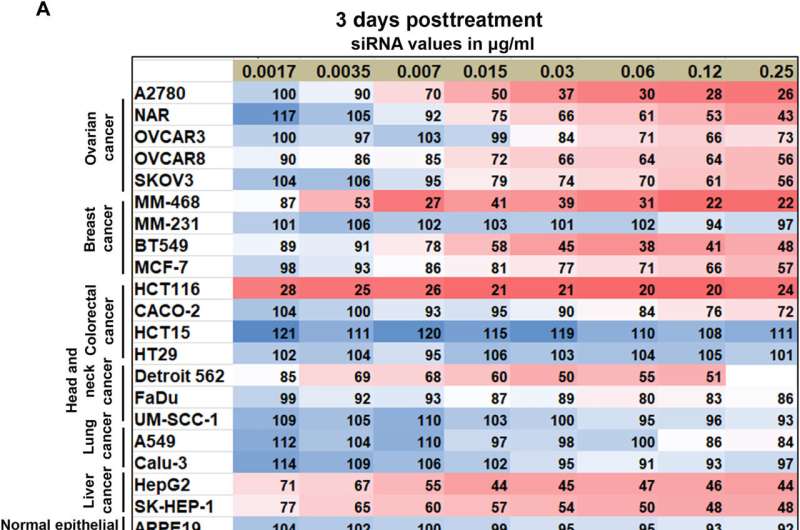
CKAP5 knockdown’s dose-dependent response in various cancer cell lines A) Methoxynitrosulfophenyl-tetrazolium carboxanilide (XTT) measurements of cell viability 72 hours and 6 days after siCKAP5 treatment Cells were treated with siCKAP5-LNP, which showed convergence when compared to a comparable grouping of siControl LNP. The XTT assay was carried out at the specified intervals. The values represent the average percentage of cells that survived when compared to cells that were given the same concentration of siControl LNP. At least three experiments were conducted, each with three technical repeats. Red boxes are used to highlight the cells where CKAP5 knockdown had no effect.
Numerous chemotherapeutic agents use mitosis inhibition to stop the cell cycle in cancer cells by examining flaws in the cell cycle machinery. Be that as it may, existing mitosis-focused chemotherapy specialists don’t separate between solid and dangerous cell lines, bringing about serious secondary effects. Therefore, locating molecular targets associated with cancer cell mitosis presents a challenge.
Microtubule-associated proteins (MAPs) Cell mitosis, where microtubule-associated proteins (MAPs) aid cells in maintaining the stability of cell dynamics, is an appealing target for effective cancer treatment. The cytoskeleton-associated protein 5 (CKAP5), which is widely expressed in numerous cells to regulate the dynamics of microtubules in human cells, is one such protein.
In this work, Chatterjee and the group screened the impact of CKAP5 quieting in strong disease cell lines and in typical non-malignant growth epithelial cell lines as a negative control. While cells with high hereditary shakiness were specifically vulnerable to CKAP5 exhaustion, a chemo-safe ovarian disease cell line showed a higher aversion to cell mediation, demonstrating the quality to be a promising restorative objective in hereditarily unsound ovarian malignant growth cell lines.
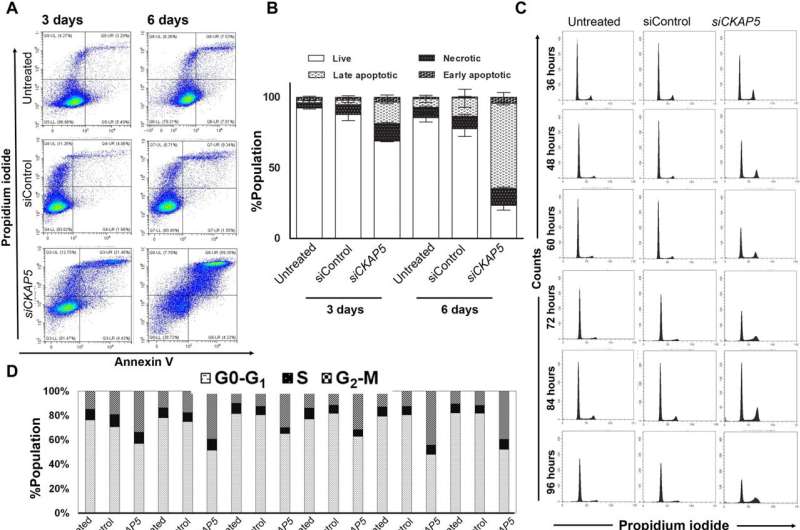
Mechanism by which CKAP5 silencing causes NAR cells to die. Cells were given either siControl or siCKAP5-LNPs (0.25 g/ml) in all of the experiments. Credit: Science Advances (2023). DOI: 10.1126/sciadv.ade4800
A) Necrotic and apoptotic cells in control and treated cells are depicted on a dot plot. After PI and annexin V–APC staining, cells were harvested at the indicated times and subjected to flow cytometry.
B) Measuring the apoptotic cell death quantitatively The data were analyzed using the unpaired t test and represent the average population SEM of three representative experiments (n = 2).
C) Cell cycle investigation throughout various time spans post-CKAP5 knockdown After being fixed in ethanol, the cells were harvested at the indicated times and stained with PI.
D) Post-CKAP5 knockdown quantitative measurement of the percentage of cells in various stages of the cell cycle
E) Confocal microscopy images of spindle formation in control and CKAP5 downregulated cells 48 hours after treatment With a zoom of 7, images were taken at 63 pixels. 10 m scale bars
(F) Quantitative confocal microscopy image analysis to determine the average number of bipolar spindle formations in control and CKAP5-deficient cells The percentage of treated and control cells with the indicated spindles is depicted on the graph. The unpaired t test is used to analyze the data, which is shown as the average SEM of 50 events. *** P 0.0001.
(G) Quantitative analysis of the mitotic spindle length in treated and control cells The distance between two mitotic posts was measured by the ImageJ investigation device. The unpaired t test was used to analyze the 40 events’ average spindle length. P 0.0001
( H) Turn around and record PCR examination of shaft-designated spot qualities following 48 and 72 hours of treatment. The data come from two representative experiments (n = 3) and represent the average fold change in comparison to untreated samples. ns means unimportant.
Credit: Science Advances (2023). DOI: 10.1126/sciadv.ade4800
The expression of CKAP5 in a variety of cancer cells has not yet been well documented. As a negative control, the researchers looked at the expression of this gene in non-cancer cell lines as well as a panel of solid cancer cell lines, including ovarian, colorectal, lung, and head-and-neck. Cryo-electron microscopy was used to examine the results of the team’s use of SiRNA-encapsulated lipid nanoparticles created through microfluidic mixing to produce uniformly sized structures.
Of the cell lines tried, they noted eight that were impervious to CKAP5 hushing, including typical or solid cell lines, which implied that typical cells didn’t respond to CKAP5 hereditary downregulation. Gene silencing was a sensitive treatment for all ovarian cancer cell lines. As a result, the team only looked into this lineage for future research. Although the team downregulated the gene of interest in all cell lines, CKAP5 depletion only affected cancer cells with high genetic instability.
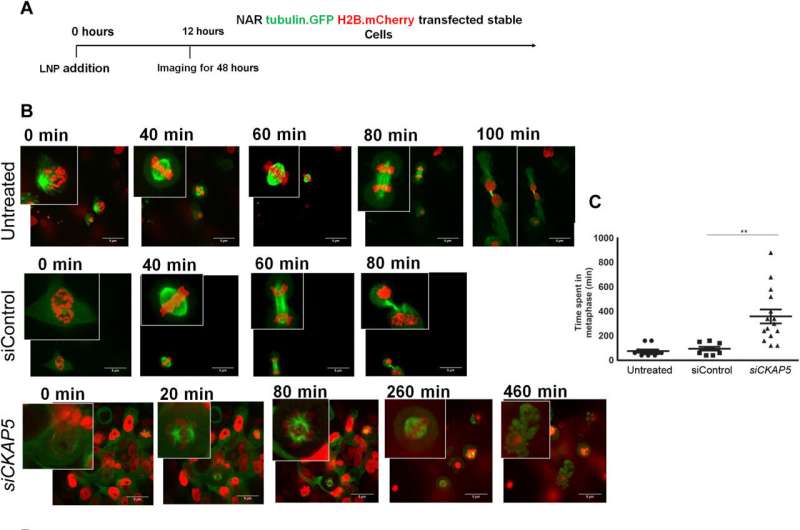
NAR cells with CKAP5 silencing are tracked live. Tubulin was used to label the cells. GFP and histone H2B.mCherry, respectively, follow the nucleus and mitotic spindle.
A) A schematic illustration of the experiment, showing when LNP addition and live-cell imaging occurred. B) Representative images of NAR cells treated with siControl or siCKAP5-LNP to follow the cell cycle Tubulin-labeled NAR cells. GFP and histone H2B.mCherry were treated with siControl or siCKAP5-LNPs (0.25 g/ml). Twelve-hour posttreatment live-cell imaging was performed by a turning-plate confocal magnifying lens. For 48 hours, images were taken every 15 minutes. 5 m scale bars (C) using a spinning disk microscope, the average amount of time spent in mitosis by various groups The unpaired t test was used to analyze the 20 cell cycle events’ average time in SEM. P = 0.0019. (D) The fate of the cells over time in the treatment and control groups The fate of each cell through the various stages of the cell cycle is represented by each bar. Credit: Science Advances (2023). DOI: 10.1126/sciadv.ade4800
CKAP5 down-regulation mechanisms: Using propidium iodide-annexin V staining, cancer biologists developed an apoptotic assay to investigate the CKAP5 down-regulation-mediated cell death mechanisms in ovarian cancer cells. They noted cell apoptosis (cell death) in the wake of transfecting hidden qualities into cell lines of interest by utilizing lipid transporters. Shaft harm in light of CKAP5 exhaustion showed a critical expansion in cells captured in metaphase, compared with control cell lines and untreated cells. Multicentric spindle formation, inhibition of the cell cycle, apoptosis, and upregulation of spindle checkpoint genes were all part of the process.
The group concentrated on cell cycle restraint in two other ovarian malignant growth cell lines to show likenesses in their ways of behaving to demonstrate the hindrance of cell reasonability. At the point when they thought about these outcomes in noncancerous ordinary epithelial cell lines, they stayed unaffected in suitability and cell cycle state. While the CKAP5 gene knockdown increased apoptotic cell death in all sensitive cancer cell lines, non-responsive cancer cell lines also did not undergo apoptotic cell death.
The specialists analyzed the systems of cell apoptosis in high-quality hushed cell lines to grasp the destiny of cells. They also used fluorescence-activated cell sorting techniques to study the lipid nanoparticle biodistribution that was localized in the liver, spleen, and tumor tissues because the silenced gene was transferred via lipid carriers.
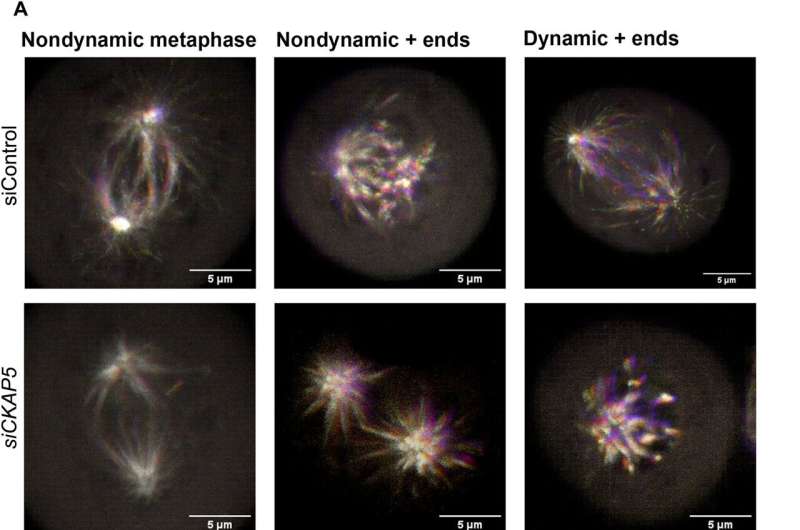
NAR cell localization and microtubule dynamics are affected by CKAP5 silencing during mitosis. A) Exemplary EB3 localization phenotypes in the control and CKAP5-silenced groups To identify the microtubule + ends, the EB3.eGFP plasmid was temporarily transfected into NAR cells. After 12 hours of transfection, cells were further transfected with siControl or siCKAP5 LNPs, and after 30 hours of siRNA-mediated silencing, live-cell imaging was carried out. A spinning disk microscope with superresolution was used to image live cells. Pictures were captured at 100. Scale bars, 5 m. For a total of 90 seconds, images were taken every second. The images are superimpositions of 91 frames, with each frame marked in a different shade on the timeframe scale. Nondynamic + closes superimpose on one another in each of the 90 casings because of their static nature and are addressed as white because of superimposition, though powerful + closes don’t superimpose in every one of the 90 edges because of their dynamic way of behaving and hence show up as a rainbow tone. In these representative superimposed images, a wider range of colors is produced by tubulin with higher dynamics. B) Quantitative portrayal of the microtubule (MT) development speed in charge and CKAP5-hushed bunch MATLAB software was used to conduct Utrack analysis on the live-cell kinetic data obtained from a spinning disk microscope. ** P = 0.0097. (C) A MATLAB-measured quantitative representation of the MT growth lifetime in the control group and the CKAP5-silenced group; (D) A MATLAB-measured quantitative representation of the MT growth length in the control and CKAP5-silenced groups. An unpaired t test is used to perform statistical analysis on the data from 10 mitotic events for each graphical analysis in (B) to (D).
After implanting the genetically modified cell lines into mice to monitor tumor growth, the team tested the effects of therapeutic gene silencing in animal models. 10.1126/sciadv.ade4800 Xenografted tumor growth and increased survival in gene-knockout animal models They randomly divided the mice into treated, control, and untreated groups eight days after implantation. They used fluorescence and luminescence imaging in vivo to study the dynamics of tumor growth after administering multiple doses. They collected tumor tissues to evaluate the tissue’s anatomy and test the effectiveness of in vivo silencing after the animal models had been treated and killed. When compared to the control groups, the animals that were given the silenced gene produced significantly less genetic expression, as was to be expected, which suggests that the experiment was successful.
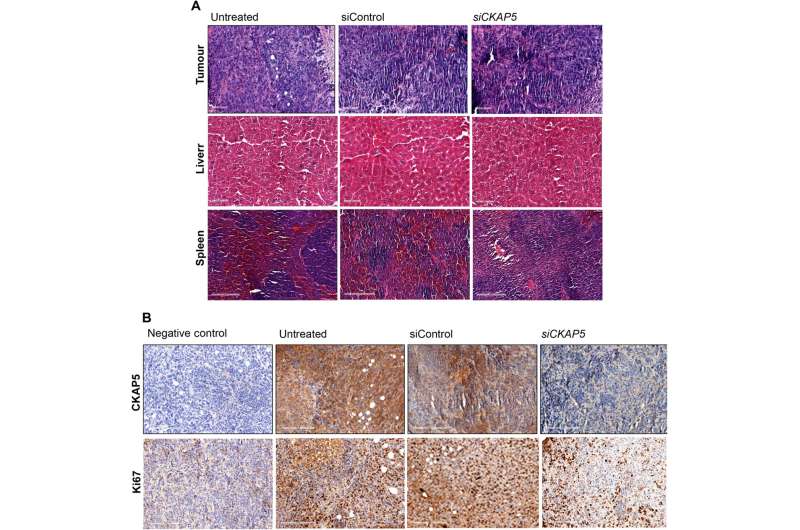
Histological examination of various tissues taken from mice treated with siCKAP5 and siControl A) Liver, spleen, and xenografted tumor tissues from untreated, siControl, and siCKAP5-LNP–treated mice were stained with H&E. 100 m are the scale bars in liver and tumor tissue. Scale bars in spleen tissue address 200 m. (B) Immunohistochemistry of ovarian disease peritoneal cancer tissue shows CKAP5 and Ki67 articulation in untreated, siControl, and siCKAP5-LNP-treated bunches. 200-meter scale bars. Credit: Science Advances (2023). DOI: 10.1126/sciadv.ade4800.
Viewpoint
For quite a long time, researchers have designated the cell cycle to investigate malignant growth treatments to conquer the chromosomal irregularities and hereditary deformities related to disease cells. Antimitotic agents that target tubulin are currently leading the pack. In any case, analysts critically try to investigate new theories, focusing on specialists. Sushmita Chatterjee and her colleagues demonstrated that tubulin function is affected by the cytoskeleton-associated protein 5 (CKAP5) gene, which affects mitotic spindle assembly. Ovarian cancer cells that are genetically unstable can benefit greatly from inhibition.
The review results feature the weakness of disease cells for CKAP5. Since ovarian malignant growth is analyzed at late stages, it will be useful to concentrate on the chance of quieting CKAP5 in late-stage ovarian disease metastasis, with suggestions to treat different tumors that metastasize and restrict to the intraperitoneal hole, including pancreatic, liver, and colorectal diseases.
More information: Sushmita Chatterjee et al, Therapeutic gene silencing of CKAP5 leads to lethality in genetically unstable cancer cells, Science Advances (2023). DOI: 10.1126/sciadv.ade4800
Deborah K. Armstrong et al, Intraperitoneal Cisplatin and Paclitaxel in Ovarian Cancer, New England Journal of Medicine (2006). DOI: 10.1056/NEJMoa052985