To dial back a dangerous atmospheric deviation, we really want to lessen ozone-harming substance emanations radically. In addition to other things, we want to manage without petroleum derivatives and utilize more energy-effective advancements.
Nonetheless, lessening discharges alone will not do what’s necessary to meet environmental targets. We should likewise catch huge amounts of the ozone-depleting substance CO2 from the air and either store it for all time underground or use it as a carbon-impartial feed material in industry. Sadly, the carbon capture innovations accessible today require a great deal of energy and are correspondingly costly.
That is the reason scientists at ETH Zurich are fostering another strategy that utilizes light. With this cycle, from now on, the energy expected for carbon capture will come from the sun. Their work has been distributed in the science of materials.
Light-controlled corrosive switch
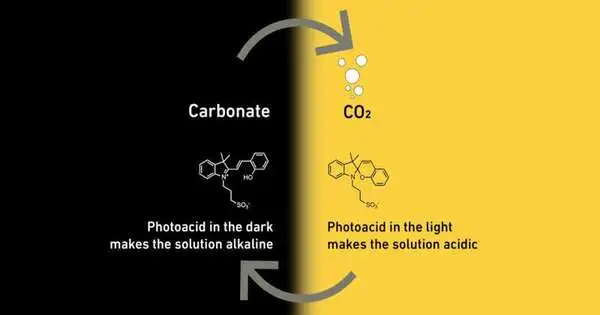
Driven by Maria Lukatskaya, teacher of electrochemical energy frameworks, the researchers are taking advantage of the way that in acidic watery fluids, CO2 is available as CO2, yet in antacid fluid fluids, it responds to the formation of salts of carbonic corrosive, known as carbonates. This substance response is reversible. A fluid’s corrosiveness determines if it contains CO2 or carbonate.
To impact the causticity of their fluid, the analysts added particles, called photoacids, to it that respond to light. In the event that such fluid is illuminated with light, the atoms make it acidic. In obscurity, they return to the first expression that makes the fluid more basic.
This is the manner in which the ETH specialists’ strategy works exhaustively: The scientists separate CO2 from the air by passing the air through a fluid containing photoacids in obscurity. Since this fluid is soluble, CO2 responds and shapes carbonates. When the salts in the fluid have collected to a critical degree, the specialists illuminate the fluid with light. This makes it acidic, and the carbonates change to CO2.
The CO2 rises out of the fluid, similarly as in a container of cola, and can be gathered in fuel tanks. At the point when there is not really any CO2 left in the fluid, the specialists switch off the light, and the cycle starts from the very beginning once more, with the fluid prepared to catch CO2.
Everything relies on the blend.
Practically speaking, nonetheless, there was an issue: the photoacids utilized are unsteady in water. “Throughout our earliest tests, we understood that the particles would disintegrate following one day,” says Anna de Vries, a doctoral understudy in Lukatskaya’s gathering and lead writer of the review.
So Lukatskaya, de Vries, and their partners examined the rot of the particle. They tackled the issue by running their response not in water but rather in the frame of mind of water and a natural dissolvable. The researchers had the option to decide the ideal proportion of the two fluids by lab tests and had the option to make sense of their discoveries on account of model estimations completed by scientists from the Sorbonne College in Paris.
For a certain something, this combination empowered them to keep the photoacid particles stable in the answer for almost a month. For another, it guaranteed that light could be utilized to switch the arrangement to and fro as expected between being acidic and being soluble. Assuming that the scientists were to utilize the natural dissolvable without water, the response would be irreversible.
Managing without warming
Other carbon capture processes are repeating as well. One laid-out strategy works with channels that gather the CO2 particles at an encompassing temperature. In this way, to eliminate the CO2 from the channels, they must be warmed to around 100°C. In any case, warming and cooling are energy-concentrated; they represent a significant portion of the energy expected by the channel strategy.
“Conversely, our interaction needn’t bother with any warming or cooling, so it requires considerably less energy,” Lukatskaya says. More than that, the ETH specialists’ new strategy possibly works with daylight alone.
“One more fascinating part of our framework is that we can go from soluble to acidic in no time and back to antacid in practically no time. That allows us to switch between carbon catch and delivery considerably more rapidly than in a temperature-driven framework,” de Vries makes sense of.
With this review, the analysts have shown that photoacids can be utilized in the research facility to catch CO2. Their subsequent stage en route to showcase development will be to increase the security of the photoacid atoms. They likewise need to research the boundaries of the whole cycle to upgrade it further.
More information: Anna de Vries et al, Solvation-Tuned Photoacid as a Stable Light-Driven pH Switch for CO2 Capture and Release, Chemistry of Materials (2023). DOI: 10.1021/acs.chemmater.3c02435