Scientists from the College of Twente, as a team with Shell, fostered another component that makes the transformation of carbon dioxide into carbon monoxide, which is a fundamental feedstock in the development of synthetic substances.
Under the auspices of the High Level Exploration Place Substance Building Blocks Consortium (Circular Segment CBBC), the scientists published their findings in the journal ACS Energy Letters.Their distribution was likewise chosen for the cover pictures of a similar diary. UT Ph.D. understudy and lead creator Sobhan Neyrizi states, “With the clever particles planned in our examination, we could enhance another pathway for CO2 transformation.”
The emanation of carbon dioxide is viewed as one of the significant reasons for a dangerous atmospheric depletion. One potential arrangement is the electrochemical change of carbon dioxide into additional valuable atoms, for example, carbon monoxide, formic acid, and hydrocarbons, which are significant intermediates in fuel and substance creation. In any case, the energy level of the response is still excessively high.
“Our molecules operate as co-catalysts, greatly reducing the energy needs of the reaction. We could potentially provide design principles for the future production of more efficient molecules.”
Sobhan Neyrizi and his fellow researchers
New pathway
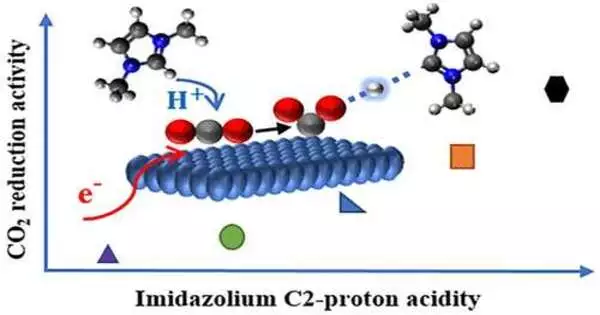
The new atoms that Sobhan Neyrizi and his kindred scientists have created can help with the electrochemical change of carbon dioxide.
“Our particles go about as co-impetuses that decrease the energy requests of the response generally,” says Neyrizi. The scientists contend they can utilize these particles to develop another pathway for the response. “We could also propose plan standards for the advancement of additional productive atoms in the future.”
100 percent effectiveness
In electrochemistry, electrons are utilized as a modest energy source. Be that as it may, moving electrons to carbon dioxidestwhich is the key step required for the changeectrons to carbon dioxide—which is the key step required for the change—requires an excessive amount of energy. The energy required can be significantly reduced by constantly transferring protons and electrons to carbon dioxide particles.
Credit: University of TwenteThe planned co-impetuses make this synchronous exchange conceivable on a surface of gold. “We could achieve 100 percent efficiency for the transformation, which means that all electrons we put into the response are used,” Neyrizi explains.
More information: Sobhan Neyrizi et al, What It Takes for Imidazolium Cations to Promote Electrochemical Reduction of CO2, ACS Energy Letters (2022). DOI: 10.1021/acsenergylett.2c01372
Journal information: ACS Energy Letters