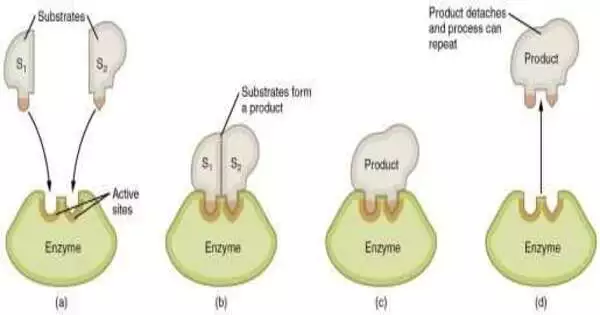
The proteins found in living creatures have great reactant power. Because of proteins, the compound responses that support life happen a great many times quicker than they would happen without them. Proteins accelerate responses by assisting with bringing the actuation energy required down to begin them, yet for over 70 years, how compounds accomplish this has been the subject of extreme discussion.
Dr. Peak Savidge, teacher of pathology and immunology at Baylor College of Medicine and Texas Children’s Microbiome Center, and his partners are altering the method for checking this old contention out. In their work distributed in Chemical Science, they examined the likenesses and contrasts between the two systems presently under banter by portraying synergist responses at an itemized sub-atomic level.
“At present time, two significant different response systems are proposed to make sense of enzymatic reactant power,” Savidge said. “One suggests that proteins bring down the response’s enactment energy through adjustment of change states (TS) and the other that they do it by weakening the ground state (GS) of compounds. The ongoing thought is that these systems are totally unrelated.”
“One theory holds that enzymes reduce the activation energy of a process by stabilizing transition states (TS), while another holds that enzymes do so by destabilizing the ground state (GS). According to current thinking, these processes are mutually incompatible.”
Dr. Tor Savidge, professor of pathology and immunology
First creator Dr. Deliang Chen at Gannan Normal University in China and his partners adopted a hypothetical strategy, thinking about past discoveries from the Savidge lab showing that the noncovalent connections of substrates and proteins with water are significant regarding the system of the enzymatic responses.
“In an organic climate you need to think about the water — that it will impede the complex nuclear connections happening in the protein’s dynamic site. We want to think about every one of them to comprehend where precisely you want to have electrostatic connections that will lean toward that enzymatic cycle,” Savidge said. “At the point when you think about that, you can comprehend how these systems are working.”
Their examinations drove the group to propose a new thing: that TS and GS are not so unique all things considered. They utilize a comparable nuclear system to help the enzymatic response forward. The system includes water in changing the charge of significant deposits inside the synergist site such that leans toward the development of a vigorously good express that drives the enzymatic response to happen.
“The significant, new point this isn’t the way this is accomplished yet when it is accomplished,” Savidge said. “We have shown that in adjustment of change expresses, the charges that drive the response forward are framed before the substrate enters the dynamic site. While in the destabilization ground express this likewise happens, yet after the substrate enters the dynamic site.”
The analysts likewise suggested that the normal system among TS and GS is general; it tends to be applied to numerous enzymatic responses.
Their discoveries have significant ramifications not exclusively to assist researchers with better grasping the reactant force of proteins, yet in addition for useful medication plan applications.
“We utilize our discoveries to more profoundly investigate microbial enzymatic catalysis in various conditions and to plan fake proteins,” Savidge said.
Yibao Li, Xun Li, Xiaolin Fan, at Gannan Normal University, and Xuechuan Hong at Wuhan University School of Pharmaceutical Sciences additionally added to this work.
More information: Deliang Chen et al, Key difference between transition state stabilization and ground state destabilization: increasing atomic charge densities before or during enzyme–substrate binding, Chemical Science (2022). DOI: 10.1039/D2SC01994A
Journal information: Chemical Science