Throughout the course of recent years, the development of long-haul impacts related to the coronavirus has prompted an expanded center around an infection with comparative trademarks and side effects: myalgic encephalomyelitis/ongoing fatigue syndrome (ME/CFS). Two examinations distributing February 8 in the diary, Cell Host and Microorganism, are investigating ME/CFS as it connects with the microbiome and the metabolites that microbial species produce.
The two examinations tracked down the fact that ME/CFS is related to decreased levels in the gastrointestinal microbiome of microorganisms known to deliver the unsaturated fat butyrate. These microbiome disturbances could explain, to some extent, how the safe framework becomes upset in individuals with ME/CFS.
“It’s essential to take note of the fact that this examination shows a relationship, not a causal link, between these microbiome changes and ME/CFS,” says Julia Gracious, an academic administrator at the Jackson Research Center and senior creator of one of the two papers. “In any case, these discoveries are the preface to numerous other robotic trials that we desire to do to learn more about ME/CFS and its fundamental causes.”
“This exploration shows that there are hearty bacterial marks of stomach dysbiosis in people with ME/CFS,” says Brent L. Williams, an associate teacher at Columbia College and senior creator of the other paper. “It assists with developing this developing field of examination by pinpointing the underlying and practical aggravations in the microbiome in an ongoing illness that influences the personal satisfaction of millions of individuals.”
“This study found that those with ME/CFS have strong bacterial fingerprints of gut dysbiosis. It contributes to the advancement of this expanding field of study by identifying structural and functional changes in the microbiome in a chronic condition that impairs the quality of life for millions of individuals.”
Brent L. Williams, an assistant professor at Columbia University.
ME/CFS is an ongoing, complex, and foundational infection related to neurological, immunological, autonomic, and energy digestion dysfunctions. It has been perceived for quite a long time, yet its causes remain ineffectively comprehended. It is widely assumed that, like the long Coronavirus, it is triggered by susceptibility to infections or other irresistible specialists.
One thing that makes ME/CFS challenging to study is that it will, in general, be heterogeneous—nnot all individuals with the illness have similar clinical histories or side effects. Both exploration groups say that’s the reason it’s vital to do studies like these that examine information from countless patients. The microbiome has as of late emerged as a possible supporter of and biomarker for ME/CFS, making it essential to study.
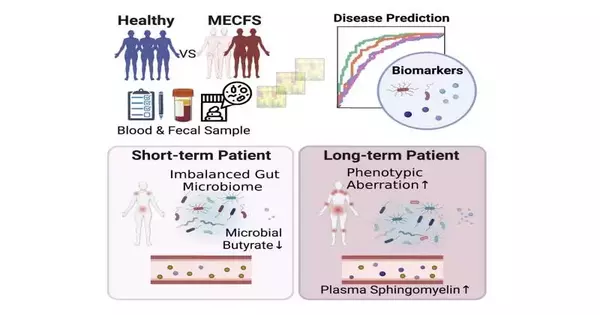
Goodness’ review used shotgun metagenomics to compare microbiome tests from people with transient ME/CFS (those who had symptoms for less than four years; 74 patients) and long-term ME/CFS (those who had symptoms for more than ten years; 75 patients), as well as 79 age- and sex-matched sound controls. The specialists also observed plasma tests from the members. The patients were being treated at the Bateman Horne Center in Salt Lake City, Utah, which has a longstanding coordinated effort with individuals from the Jackson Lab.
The examination showed that patients with transient illnesses had various changes to their microbiomes concerning variety. Most notably, they had a dearth of organisms known to produce butyrate. Butyrate is important for maintaining the trustworthiness of the stomach hindrance and is also known to play an important role in adjusting the resistant framework.
Conversely, those with long-haul illnesses had stomach microbiomes that had been restored and were more like the sound controls. Nonetheless, those members had collected various changes in the metabolites in their blood plasma, including a considerable number of those connected with the safe framework. They also had differences in the levels of specific types of safe cells and the sound controls.
Williams’ review utilized shotgun metagenomic sequencing to take a gander at the microbiomes of 106 individuals with ME/CFS and 91 controls that were matched for age, sex, geography, and financial status. This study was embraced by an interdisciplinary, multi-institutional examination bunch, the Middle for Answers for ME/CFS, and enlisted patients from five distinct locales across the US, which assisted with controlling for microbiome contrasts that might be available in various geographic areas.
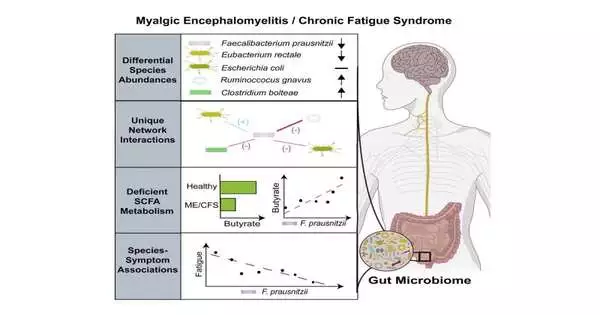
This concentrate also looked at microbial species levels in the stool.It did not include plasma investigation; however, this gathering has proactively distributed plasma metabolomics examinations from their partner elsewhere.It took a gander at metabolites in the stool, which exhibited diminished degrees of butyrate metabolites in ME/CFS.
The review from the Columbia group tracked down huge connections between the seriousness of weakness side effects and levels of explicit types of stomach microscopic organisms—sspecifically the butyrate-delivering bacterium Faecalibacterium prausnitzii. It also discovered a higher overall number of microscopic organisms in the stool and changes in bacterial species collaborations in ME/CFS patients.
More research is needed before these discoveries can be directly applied to new medicines, but experts believe they will help with the development of new symptomatic apparatuses and the development of better animal models.
“While these findings do not unequivocally demonstrate causative links between aggravations in the microbiome and side effects, these microbiome-side effect connections present potentially significant, manipulatable focuses for future restorative preliminary studies,” Williams says.”These preliminary studies may focus on dietary, probiotic, prebiotic, or synbiotic mediations and provide direct evidence that stomach microorganisms influence long-term side effects.”
Gracious notes that her future research will aid in the segmentation of patients based on the elements of their illness, including those with conditions frequently associated with ME/CFS, such as peevish gut disorder and neuroinflammatory messes.”This will assist us with pinpointing explicit microbial and metabolomic factors that are related to this illness,” she says.
Williams intends to additionally examine his discoveries in creature models. “A manageable mouse model focusing on the stomach microbiome aggravations discovered in ME/CFS would provide a significant tool to assess causal hypotheses, systems, and medicines,” he says.
More information: Julia Oh, Multi-‘omics of gut microbiome-host interactions in short- and long-term Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS) patients, Cell Host & Microbe (2023). DOI: 10.1016/j.chom.2023.01.001