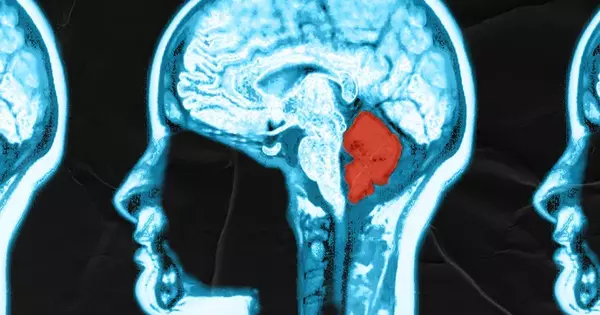
The cerebellum is a brain area involved with motor coordination and balance, but it also plays a role in other cognitive activities. It includes an enormous number of neurons, accounting for roughly 10% of the total amount of neurons in the brain. These neurons make extensive and exact connections with one another, allowing for delicate and precise movement and coordination control.
Although neuroscientists have long assumed that Purkinje cells had just one main dendrite that connects to a single ascending fibre from the brain stem, new research demonstrates that virtually all Purkinje cells in the human cerebellum contain numerous primary dendrites.
Santiago Ramón y Cajal, a Spanish scientist, was awarded the Nobel Prize in 1906 for his pioneering investigations of the microscopic structures of the brain. His famous illustrations of Purkinje cells in the cerebellum depict a forest of neuron structures, with several enormous branches growing from the cell body and breaking into lovely, leaf-like patterns.
Although these early drawings showed several dendrites extending from the cell body, neuroscientists now believe that Purkinje cells have only one principal dendrite that connects to a single climbing fibre from the brain stem. However, new research released this week in Science by the University of Chicago demonstrates that Cajal’s drawings were correct all along – nearly all Purkinje cells in the human cerebellum have numerous main dendrites.
You get a sense for how much this was a prevailing idea in the field because anatomically, they are referred to as the ‘primary’ dendrite of a cell. So, even the description of the structure of these cells is based on that mouse prototype that has one dendrite you can call a primary dendrite.
Silas Busch
Further research in mice revealed that approximately 50% of their Purkinje cells have this more complex structure, with 25% receiving input from several climbing fibres that connect with distinct principal dendritic branches. Experiments using live mice to monitor cell activity indicated that the major branches can be engaged separately, responding to distinct environmental cues.
“The more you work with a certain prototype of a cell in your mind, the more you accept it,” said Christian Hansel, PhD, Professor of Neurobiology at UChicago and the study’s senior author, referring to the canonical model that Purkinje cells have one primary dendrite that connects to one climbing fibre.
“These drawings by Cajal have been around since the 1900s, so we definitely had enough time to pay attention, but only now with this quantitative analysis do we see that it’s almost universal that human cells have multiple full dendrites each, and we can see that it makes a qualitative difference too.”
Rewriting a textbook idea
The cerebellum (from the Latin for ‘small brain’) is located at the base of the cranium, directly above the spinal cord’s connection. Since French physician Jean Pierre Flourens first described the cerebellum’s function in 1824, scientists assumed that it was solely responsible for coordinating movement and muscular activity. However, technological advances have revealed that the cerebellum also plays a significant role in processing input about the body’s internal and external environments, including sensations of proprioception and balance.
Cerebellar Purkinje cells function similarly to huge antennae, receiving hundreds of inputs and transmitting a wide range of contextual information from the rest of the body. These signals are then combined with a prediction-error signal to indicate a discrepancy between the context and the brain’s anticipation. This error signal is produced by nerve fibres that ascend from the brain stem and link with Purkinje dendritic structures. These nerves are aptly referred to as “climbing fibres.”
The standard understanding of these connections has been that each Purkinje cell has one primary dendrite that branches from the cell body and connects with one climbing fiber, forming a single computational unit. Belief in this one-to-one relationship between climbing fibers and Purkinje cells, a central dogma in the field that can be found in every neuroscience textbook, largely comes from studies on rodents, which do primarily have the single dendrite configuration.
Many previous studies of these structures have concentrated on tiny numbers of cells, so for this new study, Silas Busch, a PhD student in Hansel’s lab and first author on the publication, began by looking at thousands of cells from both human and mouse tissue. He labelled Purkinje cells in thin slices of cerebellum using immunohistochemistry, a targeted antibody-based staining approach. He next classified the anatomy of all the cells he could see and discovered that more than 95% of human Purkinje cells had several main dendrites, but in mice, the figure was much closer to half.
“You get a sense for how much this was a prevailing idea in the field because anatomically, they are referred to as the ‘primary’ dendrite of a cell,” Busch said. “So, even the description of the structure of these cells is based on that mouse prototype that has one dendrite you can call a primary dendrite.”
This significant species variation in one of the most evolutionarily conserved brain areas shared by mammals and other vertebrates prompted Busch and Hansel to wonder if there might be a functional benefit to having many main dendrites rather than just one. Their first suspect was the climbing fibre, which had an advantageous one-to-one interaction and was intimately entangled with the principal dendrite.
Using sections of mouse cerebellum that contained still living cells, Busch injected the cells with dye to see their branches and then stimulated climbing fiber inputs. He discovered that 25% of cells with multiple primary dendrites received multiple climbing fibres, rewriting the textbook notion that each Purkinje cell receives just one climbing fibre input, but cells with a single primary dendrite did not.
Walking mice and wiggling whiskers
Encouraged by this finding that a sizable portion — albeit a minority — of Purkinje cells with multiple primary dendrites also received input from multiple climbing fibers, Busch conducted a series of experiments in living mice to see if it led to functional differences in the live mouse. First, he injected a fluorescent calcium indicator dye into the cerebellum and implanted a small glass window so he could later observe the flow of calcium into the Purkinje cell dendrites.
By restraining the mouse’s head under a microscope while it ran on a treadmill, he could measure calcium flow that indicated when a climbing fiber is providing a strong input to the cell. In cells with one primary dendrite, high-resolution images showed that the activity signal was uniform across its structure; in cells with multiple primary dendrites, he could detect activity on each side occurring at different times, meaning that one dendrite could be activated by its climbing fiber while the other dendrite in the same cell was not.
Busch then sought to explore if he could isolate individual climbing fibre activity using a more precise stimulus: the mouse’s whiskers. Busch had to sedate the mice for this experiment (“I don’t know if you’ve ever tried to stimulate individual whiskers in an awake mouse, but it’s really hard,” he remarked). Busch inserted individual whiskers into a small glass tube and moved them back and forth while the mice were sleeping. He could also witness activity in Purkinje cell dendritic branches, implying that individual climbing fibres were signalling information from individual whiskers to individual dendrites.
Finally, for a more realistic setting, Busch tested conscious mice with various stimuli such as light flashes, sounds, and air puffs on the whisker pad. Again, he noticed variances in Purkinje cells. In rare cases, one branch may prefer one stimulus over another, so it may be particularly sensitive to light but not sound. The other branch may then respond preferentially to sound but not light.
“This happened in a minority of cells because mice have fewer cells with multiple branches and not all of them get multiple climbing fibres, but the presence of this effect was very interesting,” Busch said. “It confirmed this idea that the two climbing fibre inputs will have different functional purposes representing different information.”