There is a consistently present battle to diminish carbon-based energy sources and supplant them with low- or no-carbon choices. The method involved with parting water could be the goal.
Hydrogen creation is a basic, safe, and viable technique to deliver more energy than fuel can through the straightforward process of parting water. Gathering energy along these lines, rather than depending intensely (or by any means) on carbon-based energy sources, is progressively becoming the norm. Specialists have tracked down a strategy to utilize progress metal sulfides, similar to tin (Sn), cobalt (Co), and iron (Fe) on nickel froth, to foster non-valuable metal electrocatalysts for use in practical and naturally capable water parting.
The specialists have distributed their outcomes in nanoexploration energy.
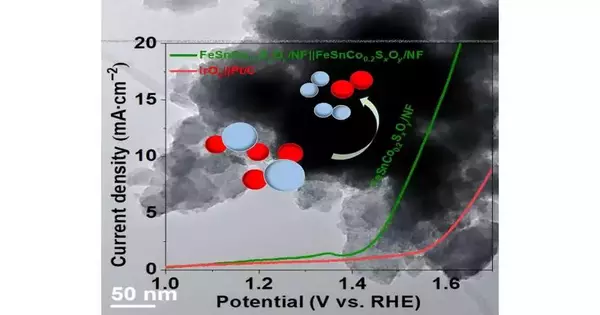
To find lasting success in this carbon-lessening adventure, a few responses should be balanced out for this cycle. The star of the review is FeSnCo0.2SxOy/NF, which can go about as both an anode and cathode during the time spent parting water at a low voltage.
“Improving the OER stability of transition metal sulfides is critical in order for them to be used as bifunctional HER and OER catalysts for reversible hydrogen fuel cells.”
Jingqi Guan, author and researcher of the study.
The two responses of concern here are oxygen development responses (OER) and hydrogen advancement responses (HER). OER produces O2 through a synthetic response from water. HER yields H2 from a two-electron move response. The subsequent H2 is valuable as fuel. Utilizing both of these responses is great for making a bifunctional electrocatalyst. Electrocatalysts can be characterized as impetuses (or response starters) that have capability at terminal surfaces, which are surfaces that can convey an electrical flow.
HER has ended up being steady at 55 hours of nonstop use and, furthermore, requires a lower overpotential than OER. Overpotential is the difference in how much energy is required for a given stimulus to work.
Sadly, OER steadiness isn’t where it should be. This is somewhat because of the additional step engaged with the electron move, yet in addition, in light of the fact that the electrolytes they are capable of are regularly unforgiving. While OER is steady with nonstop utilization for close to 70 hours, its movement diminishes with more cobalt content.
“It is significant to further develop the OER steadiness of change metal sulfides so they can be utilized as bifunctional HER and OER impetuses for reversible hydrogen energy components,” said Jingqi Guan, creator and analyst of the review.
OER likewise has a higher overpotential than HER. With a higher measure of energy expected to prompt the impetus into activity, OER can be more “troublesome.” In any case, the blend of iron, tin, and cobalt on nickel froth flaunts some improvement in bifunctional soundness and both HER and OER action.
The mix of these metals and the heterostructural interfaces can change the dispersion of the electrons across the electrolyte surface. “Heterostructural” here alludes to a semiconductor that can have a change in substance creation in view of the position the two synthetics are in. In this example, it is a sulfide/oxyhydroxide team.
Indeed, even the circulation of electrons assists with expanding the pace of charge movement all through the entire design, which then, at that point, advances the exchange of electrons. Because of the idea of this semiconductor, expanding soundness would normally work on general action and capability.
In general, these progress metals synergistically affect one another, particularly while going through HER. This impact makes them an ideal contender for the fundamental test proposed by specialists: lessening carbon-based energy sources.
However, the outcomes were exceptionally encouraging, and there are generally steps that can be taken in the future to consummate a cycle. Finding an impetus that limits the overpotentials can diminish the energy input expected to catalyze the response. Also, guaranteeing the electrocatalysts created are sufficiently sturdy to be utilized economically and can endure extended periods of nonstop utilization with no evil impacts is basic to the drawn-out progress of the heterostructural interfaces.
More information: Siyu Chen et al. Interface engineering of Fe-Sn-Co sulfide/oxyhydroxide heterostructural electrocatalyst for synergistic water splitting, Nano Research Energy (2023). DOI: 10.26599/NRE.2023.9120106