An exploration group led by Teacher Jong-Beom Baek and his group in the School of Energy and Compound Designing at UNIST has accomplished a huge leap forward in battery innovation. They have fostered a creative technique that empowers the protected combination of fluorinated carbon materials (FCMs) utilizing polytetrafluoroethylene (PTFE) and graphite.
Fluorinated carbon materials definitely stand out because of their uncommon solidity, ascribed to serious areas of strength for the F holding—the most grounded among carbon single bonds. In any case, customary techniques for fluorination include profoundly poisonous reagents, for example, hydrofluoric corrosive (HF), making them unacceptable for useful applications.
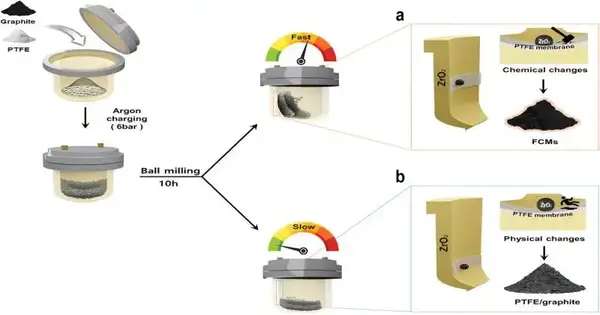
In this review, the exploration group presented a clear and generally safe methodology for a versatile combination of FCMs through mechanochemical depolymerization of PTFE—a regularly involved compound tracked down in ordinary things—and fracture of graphite. By using ball-processing procedures that actuate both mechanical and compound responses, they effectively delivered FCMs with essentially further developed execution compared with graphite.
“This study demonstrates not only safe fluorination methods, but also the broader potential of solid-phase reactions.”
Boo-Jae Jang, a researcher in the School of Energy and Chemical Engineering at UNIST.
The utilization of dangerous mixtures like fluorine gas, or HF, in regular carbon fluoride creation raises security concerns, expanding fabrication costs related to severe wellbeing measures. To address these difficulties, Teacher Baek’s group concocted a strong stage fluorination strategy utilizing PTFE, an inactive polymer known for its security under barometrical circumstances and innocuousness when consumed orally.
Through tests, it was seen that exposing PTFE to more energy than it can endure prompts sub-atomic chain breakage and revolutionary development—starting a response bringing about the creation of carbon fluoride edifices. These buildings then, at that point, stick to the surface and edges of graphite particles during the resulting processes.
The subsequent FCMs exhibited an unrivaled capacity limit and electrochemical solidity compared with customary graphite anodes. At a low charging pace of 50 mama/g, the FCMs showed capacity limits 2.5 times higher (951.6 mAh/g) than graphite, while at a high charging pace of 10,000 mama/g, their capacity limit was ten times higher (329 mAh/g). Surprisingly, even after in excess of 1,000 charge/release cycles at a pace of 2,000 Mama/g, the FCMs held 76.6% of their underlying limit, compared with just 43.8% for graphite.
“This review features safe fluorination strategies as well as the more extensive capability of strong stage responses,” expressed Boo-Jae Jang, a specialist in the School of Energy and Substance Designing at UNIST.
“This examination prompts us to rethink materials that are normally tracked down in our environmental factors,” added Teacher Baek. He further stressed the meaning of understanding strong stage responses as it opens ways to creating novel materials that were already neglected.
The review discoveries have been distributed in Cutting-edge Practical Materials.
More information: Boo‐Jae Jang et al, Direct Synthesis of Fluorinated Carbon Materials via a Solid‐State Mechanochemical Reaction Between Graphite and PTFE, Advanced Functional Materials (2023). DOI: 10.1002/adfm.202306426