Parasitic infections, as unpleasant as they sound, are far more common than you may believe. They are frequently to blame for the various health issues we face. A parasitic infection can have a negative impact on your physical and mental health, causing everything from digestive problems to post-traumatic stress disorder.
Toxoplasmosis is a common but usually non-fatal parasitic infection caused by tainted food or water. While most people infected with Toxoplasma gondii (T. gondii), the parasite that causes toxoplasmosis, will have very mild or no symptoms, the parasite can live in the body for long periods of time, possibly even a lifetime.
New research into the effects of common parasite infection on human behavior could aid in the development of treatments for schizophrenia and other neurological disorders. T. gondii currently infects 2.5 billion people worldwide, causing Toxoplasmosis.
Lower levels of norepinephrine may be linked to behavioral changes in those infected with T.gondii. Norepinephrine also has anti-inflammatory properties. Neuroinflammation and norepinephrine have both been linked to a variety of psychiatric disorders, including schizophrenia and ADHD.
New research into how a common parasite infection alters human behavior could help the development of treatments for schizophrenia and other neurological disorders.
According to scientists, those infected with T. gondii, which currently infects 2.5 billion people worldwide and causes the disease, exhibit behavioral changes. Toxoplasmosis may be associated with decreased levels of norepinephrine, a chemical released in the brain as part of the stress response. Norephinephrine also regulates neuroinflammation, which is the activation of the brain’s immune system in response to infection.
Neuropsychological disorders such as schizophrenia, Alzheimer’s disease, and ADHD have been linked to norepinephrine and neuroinflammation. Although T. gondii infection is usually asymptomatic in humans, it can cause headaches, confusion, and seizures in others, as well as increased susceptibility to schizophrenia and, can be fatal in immunocompromised patients.
T. gondii can only reproduce sexually in cats. It produces cysts, which are excreted in the cat’s feces. It enters new hosts through ingestion of anything contaminated by these cysts, such as water, soil, or vegetables; blood transfusions from unpasteurised goat’s milk; eating raw or undercooked meat; or transmission from mother to fetus.
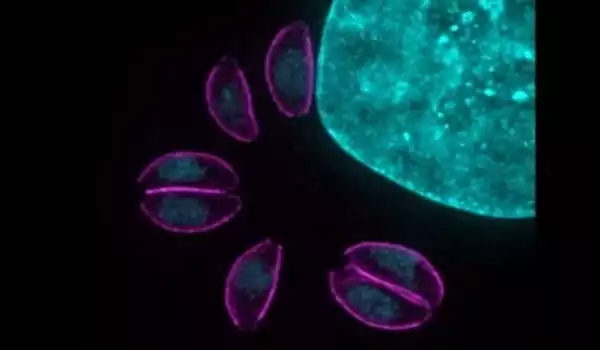
Toxoplasma gondii is a protozoan parasite with a diverse host range and a complex life cycle. Through a combination of carnivory and vertical transmission, the asexual portion of its life cycle can be sustained indefinitely in a variety of intermediate hosts. T. gondii, on the other hand, only produces gametes in felids after predation on infected intermediate hosts. The parasite alters the behavior of its intermediate hosts by reducing their innate fear of cat odors, potentially increasing the likelihood that the infected host will be devoured by the definitive host.
After a few weeks, the infection goes dormant, and cysts form in the brain. They can stay there for many years, if not forever. Infection reduces the brain’s immune response regulator norepinephrine during this stage.
The mechanisms by which the parasite affects brain function are still unknown. However, new research from the Universities of Leeds and Toulouse suggests that the parasite’s ability to reduce norepinephrine disrupts control of immune system activation, allowing for an overactive immune response that may alter the host’s cognitive states.
The findings were published in Trends in Immunology as Noradrenergic Signaling and Neuroinflammation Crosstalk Regulates Toxoplasma gondii-Induced Behavioral Changes.
Glenn McConkey, Associate Professor of Heredity, Disease, and Development at the University of Leeds’ School of Biology, who led the study, stated: “Our discovery bridges the gap between two opposing theories about how Toxoplasma alters host behavior, and it may be applicable to other nervous system infections. One school of thought believes that changes in behavior are caused by the immune response to infection, while the other believes that changes are caused by altered neurotransmitters.”
“This research will contribute to the critical need for understanding how brain inflammation is linked to cognition, which is critical for the future development of antipsychotic treatments.”
This research is important beyond toxoplasmosis because T. gondii is related to parasites that cause malaria and life-threatening diarrheal diseases. Understanding T. gondii’s transport mechanism will also provide insight into these parasites. Scientists can use their understanding of how a parasite like T. gondii completes cargo transport functions to develop drugs that will kill the parasite.





