The improvement of profoundly effective energy-stockpiling gadgets that can store environmentally friendly power is vital to a feasible future. In this day and age, strong state battery-powered lithium ion (Li+) batteries are the cutting edge. Yet, lithium is an uncommon earth metal, and society’s reliance on the component is probably going to prompt a fast decrease in assets and ensuing cost climbs.
Magnesium particle (Mg2+)-based batteries have picked up speed as an option in contrast to Li+. The world’s hull holds adequate magnesium, and Mg2+-based energy gadgets are said to have high energy densities, high security, and minimal expense. Yet, the wide use of Mg2+ is restricted by its unfortunate conductivity in solids at room temperature. Mg2+ has unfortunate strong state conductivity on the grounds that divalent positive particles (2+) experience solid connections with their adjoining negative particles in a strong gem, blocking their movement through the material.
“In this study, we made use of a group of substances known as metal-organic frameworks (MOFs). Due to the very porous crystal structures of MOFs, the incorporated ions can move through them effectively. Acetonitrile, a “guest molecule,” was also introduced into the pores of the MOF in this case, and it was successful in significantly increasing the conductivity of Mg2+.”
Junior Associate Professor Masaaki Sadakiyo of TUS
An examination group from Tokyo University of Science (TUS) recently overcame this obstacle.In their new review, distributed online on 4 May 2022 and on 18 May 2022 in volume 144 issue 19 of the Journal of the American Chemical Society, they report interestingly, a strong state Mg2+ guide with a superionic conductivity of 103 S cm1 (the edge for useful application in strong state batteries). This size of conductivity for Mg2+ guides is the most elevated answered to date. As per Junior Associate Professor Masaaki Sadakiyo of TUS, who led the review, “In this work, we took advantage of a class of materials called metal-natural systems (MOFs). MOFs have profoundly permeable gem structures, which give space for effective movement of the included particles. We also introduced a ‘visitor particle,’ acetonitrile, into the pores of the MOF, which resulted in a significant increase in Mg2+ conductivity.”The exploration group additionally included Mr. Yuto Yoshida, likewise from TUS, Professor Teppei Yamada from the University of Tokyo, Assistant Professor Takashi Toyao, and Professor Ken-ichi Shimizu from Hokkaido University. The paper was made accessible online on May 4, 2022 and was distributed in Volume 144, Issue 19 of the diary on May 18, 2022.
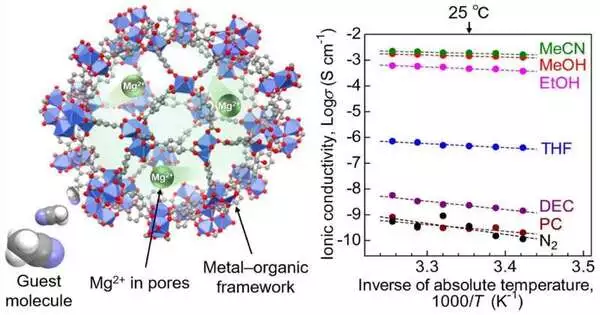
The group utilized a MOF referred to as MIL-101 as the primary system and afterward embodied Mg2+ particles in its nanopores. In the resultant MOF-based electrolyte, Mg2+ was approximately stuffed, permitting the movement of divalent Mg2+ particles. To further improve particle conductivity, the exploration group exposed the electrolyte to acetonitrile fumes, which were adsorbed by the MOF as visitor atoms.
The group then exposed the pre-arranged examples to a rotating current (AC) impedance test to gauge ionic conductivity. They found that the Mg2+ electrolyte displayed a superionic conductivity of 1.9 103 S cm1. This is the most elevated at any point in detailed conductivity for a glasslike strong holding back Mg2+.
To comprehend the system behind this high conductivity, the analysts did infrared spectroscopic and adsorption isotherm estimations on the electrolyte. The tests uncovered that the acetonitrile atoms adsorbed in the system were considered the effective movement of the Mg2+ particles through the body of the strong electrolyte.
This study’s findings not only reveal the clever MOF-based Mg2+ guide as a viable material for battery applications, but also provide fundamental insight into the development of future strong state batteries. For quite a while, individuals have accepted that divalent or higher valency particles can’t be effectively moved through a strong “In this review, we have shown that in the event that the gem structure and general climate are very much planned, a strong state high-conductivity guide is well within the research,” makes sense to Dr. Sadakiyo.
When he gets some information about the exploration gathering’s likely arrangements, he discovers that they “desire to additionally add to society by fostering a divalent guide with much higher ionic conductivity.”
More information: Yuto Yoshida et al, Super Mg2+ Conductivity around 10–3 S cm–1 Observed in a Porous Metal–Organic Framework, Journal of the American Chemical Society (2022). DOI: 10.1021/jacs.2c01612
Journal information: Journal of the American Chemical Society