A group of scientific experts and physicists at Aarhus College, in Denmark, working with a partner from the Universitat de Barcelona, in Spain, has recorded molecule-by-particle solvation interestingly. In their review, distributed in the journal Nature, the gathering planned a cycle to control sodium and xenon particles with a drop of helium at freezing temperatures to catch what they depict as previews of the solvation interaction after some time. Consolidated, these produce a film portraying the activity. An exploration preparation for the work has been distributed in a similar diary issue.
Solvation is the dissolution of a solute in a dissolvable solution—when salt disintegrates in water, for instance. The activity doesn’t stop in light of the fact that the solute has broken up; all things considered, the solvents keep on connecting with the material that has been disintegrated.
Earlier examination has demonstrated the way that such collaborations can be very muddled, which is the reason scientific experts need to find out about what occurs. One method for finding out is to film the activity and play it like a film. This basic idea has demonstrated to be especially troublesome, but so troublesome that it was not achieved as of not long ago by the group in Denmark.
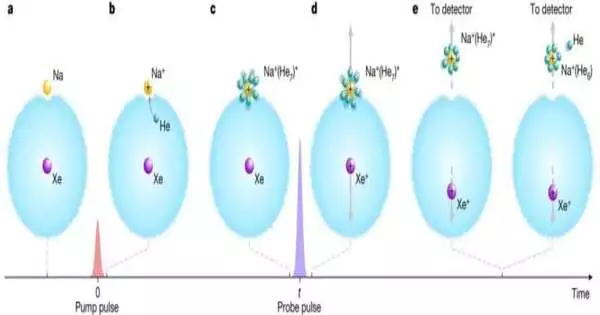
To accomplish their accomplishment, the specialists started by catching a solitary xenon molecule inside a bead of fluid helium that had been cooled to -255 °C and afterward adding a solitary sodium iota to the external edge of the drop. They terminated a short heartbeat from a laser at the sodium molecule to change it into an emphatically charged particle, setting off solvation—the helium iotas started to adhere to the sodium particle.
TDDFT reenactment of the Na+ particle solvation process. Left video: time development of the He thickness in a balance plane. The red speck addresses the Na+ particle. Right video: the strong dark line shows the roundly found middle value of the drop thickness profile around the particle (left vertical pivot). The red line shows the quantity of He iotas as an element of the distance to the particle (right vertical pivot). Credit: Nature (2023). DOI: 10.1038/s41586-023-06593-5
The group then, at that point, terminated another laser beat, this time at the xenon particle, transforming it into an emphatically charged particle. The two particles repulsed each other so much that the sodium particle, with its joined helium iotas, was pushed out of the bead and onto an indicator, which took into consideration the catch of a preview of what was happening.
The analysts then rehashed the cycle, each time standing by longer to fire the subsequent heartbeat. They had the option to make what they depicted moderate previews of the activity. Then, at that point, when they had various successive previews, they sewed them together to make a film that portrayed the solvation cycle in real life.
More information: Simon H. Albrechtsen et al, Observing the primary steps of ion solvation in helium droplets, Nature (2023). DOI: 10.1038/s41586-023-06593-5
Helium droplets help to visualize the start of ion solvation, Nature (2023). DOI: 10.1038/d41586-023-02950-6