Platelet transfusions can be expanded to be used as cell therapy for a variety of diseases. They are essential for managing bleeding and hemostatic dysfunction. The endeavors to make such cell treatments expect that scientists adjust contributor platelets to communicate helpful proteins. Be that as it may, as of now, fitting strategies to hereditarily change platelets gathered from blood contributors stay subtle.
In another review distributed in Science Advances, Jerry Leung and a group of researchers in nanomedicine, natural chemistry, and sub-atomic science at the College of English Columbia, Canada, the Hokkaido College, Japan, and different foundations in the U.S. portrayed a methodology in view of platelet-advanced lipid nanoparticles containing mRNA for exogenous protein articulation in human and rodent platelets.
Exogenous protein expression that resulted from the library of mRNA-lipid nanoparticles was not correlated with platelet activation, as the team discovered when they tested it. After being transplanted into rats, the transfected platelets maintained their hemostatic function and accumulated in areas of vascular damage, enhancing their therapeutic potential.
Platelets and hemostasis
Platelets are vital to hemostasis and are regularly bonded to reestablish hemostatic equilibrium in patients. These platelets can be extended past signs, for example, cell treatments to treat sepsis, aggravation, and joint inflammation. Hereditarily altered platelets can make new cell treatments that express restorative proteins, which can be carried out to adjust donor platelets. Existing techniques for electroporation, viral vectors, and business transfection have not had the option to alter benefactor platelets and express exogenous proteins.
Circuitous methodologies can communicate exogenous proteins in platelets or platelet-like particles by focusing on platelet-forerunner, undeveloped cells with lentiviral vectors. The donor determined platelets should be practically changed to make legitimate platelet cell treatments.
Earlier endeavors to transfect platelets with lipid nanoparticles containing mRNA have shown the chance of mRNA conveyance into the platelets, while progress in lipid nanoparticle innovation has worked on arriving at a more extensive demographic potential.
In this work, Leung and associates announced mRNA lipid nanoparticles for their ability to straightforwardly transfect giver platelets to communicate exogenous proteins. Such platelets can be adjusted with mRNA lipid nanoparticles to keep up with their capability, aggregate locally in injuries, and control homeostasis after bonding in coagulopathic rodents.
Lipid nanoparticles work with exogenous protein articulation in platelets.
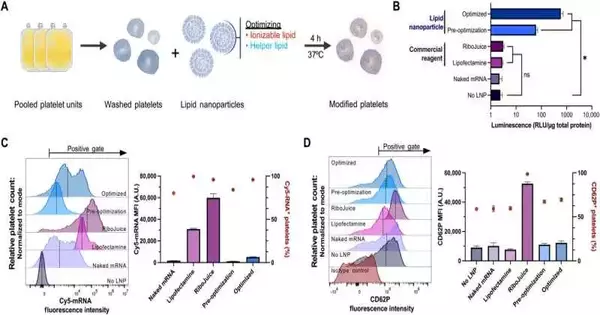
To distinguish the successful transfection strategies for platelets, the group conveyed mRNA encoding a catalyst, NanoLuc luciferase (NanoLuc), utilizing a few transfection specialists and estimating their demeanor. While NanoLuc was undetected in platelets treated with free mRNA without a transfection specialist or by utilizing business mRNA conveyance specialists, the cycle permitted the take-up of a lot of mRNA into the platelets.
An mRNA-lipid nanoparticle formulation that resembled the small interfering RNA-lipid nanoparticle clinically proven to treat hereditary amyloidosis was used by Leung and colleagues to detect NanoLuc expression. The group looked at how much platelet actuation followed mRNA lipid nanoparticle transfection to untreated platelets.
To distinguish the mRNA-lipid nanoparticle plan generally fit to ship platelets, they enhanced three significant parts: ionizable lipids, linker lipids, and the polyethylene glycol lipid. They screened 10 ionizable and two all-time cationic lipids and estimated their protein articulation, mRNA take-up, and enactment to help protein union.
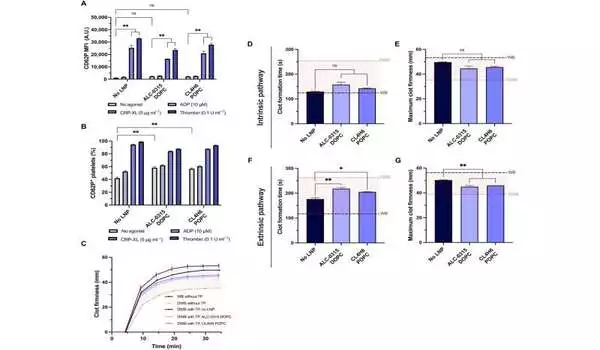
Platelets treated with LNP keep up with their capacity to enact and add to the development and immovability of blood clumps. (A and B) Platelet activation as measured by the MFI of surface CD62P levels (A) and the percentage of platelets positive for surface CD62P (B) without the use of agonists, ADP, CRP-XL, or thrombin stimulation. C) Delegate ROTEM bends, with thickening started by ellagic corrosive (n = 3). The red concealed district is the region between entire blood (WB) and weakened WB (DWB) without the extra bonding bundle (TP) added. (D to G) Measuring ROTEM cluster arrangement time (s) and greatest clump immovability (mm) for thickening actuated with ellagic corrosive through the inborn pathway (C and D) or thromboplastin by means of the outward pathway (E and F). The running lines address the immovability of WB (dim red) and DWB (light dark). Credit: Science Advances (2023). DOI: 10.1126/sciadv.adi0508
Activities of the functionalized lipid nanoparticles in the lab
To inspect how the mix of ionizable and assistant lipids has synergistic impacts to upgrade protein articulation while limiting platelet initiation, the group concentrated on two FDA-endorsed ionizable lipids. The mRNA elements played a significant role in facilitating effective exogenous protein synthesis in addition to their lipid composition. For platelet transfection and driving higher expression levels, the lipid nanoparticles containing helper lipids with a phosphocholine head and lipids with branched or unsaturated tail groups were best suited.
Of the RNA adjustments tried in this work, Leung and associates noted that unmodified uridine or pseudouridine worked with higher articulation levels of fluorescence. They then noticed on the off chance that the statement of fluorescence relied upon the level of platelet initiation or on how much RNA was conveyed, which they concentrated on utilizing a connection grid examination.
While the fluorescence articulation didn’t emphatically correspond with surface platelet levels or mRNA take-up, they noticed a gentle positive connection between how much RNA was conveyed and platelet enactment. Since NanoLuc articulation didn’t firmly relate to either surface platelet levels or mRNA take-up, the group tried the chance of affecting its appearance by initiating platelets utilizing agonists during mRNA lipid nanoparticle transfection.
Platelet treatment to show dilutional coagulopathy
Platelets invigorated with adenosine diphosphate, for example, a cross-connected collagen-related peptide or thrombin preceding the mRNA-lipid nanoparticle treatment, had fundamentally less fluorescence articulation. At the point when Leung and the group animated platelets with agonists for over two hours, they went through a significant adjustment of the transcriptome and proteome. The outcomes showed that the interpretation of exogenous mRNA didn’t need platelet enactment.
At the point when the group treated platelets with mRNA lipid nanoparticles, they kept up with hemostatic capability in vitro and showed high aversion to their physical and synthetic conditions. The group explored if the platelets would, in any case, be enacted after mRNA-lipid nanoparticle transfection and estimated their actuation state and reaction to physiological agonists.
The group tried the limit of transfected platelets to hold their capability to add to the solidity and pace of clump development, utilizing a model of rotational thromboelastometry and an ex vivo model to test platelet movement in the entire blood. The scientists demonstrated dilutional coagulopathy involving weakened entire blood and arranged platelets in a bonding bundle.
At the point when they joined the bonding bundle with weakened entire blood to show the illness as it happens in a patient, they noticed that the lipid nanoparticles didn’t influence platelet coagulopathy in vitro. Also, the group investigated the declaration of platelets transfected with mRNA lipid nanoparticles communicated with NanoLuc, coursed and restricted into twisted locales after bonding into coagulopathic rodents.
Viewpoint
Along these lines, Jerry Leung and partners designated the conveyance of atoms and cell treatments into vasculature locales of interest by utilizing the normally able platelets that can intrinsically play out this undertaking. The group created platelet-upgraded lipid nanoparticles and mRNA for effective protein articulation while introducing platelet round capability and nearby gathering at the vasculature site of interest.
It is feasible to accomplish the conveyance of nucleic acids and exogenous interpretation utilizing platelet-upgraded mRNA-lipid nanoparticles to expand and design platelets for different clinical applications. Such giver platelets designed with mRNA-lipid nanoparticles can treat intense draining issues with more extensive applications in oncology. These platelets bonded with streamlined mRNA-lipid nanoparticles are practically transfusable and can amass at the vasculature site for successful platelet treatments to adjust hematological issues.
More information: Jerry Leung et al. Genetically engineered transfusable platelets using mRNA lipid nanoparticles, Science Advances (2023). DOI: 10.1126/sciadv.adi0508