As of late, an exploration group has distributed another concentrate on starting high-voltage multi-electron responses in NASICON cathodes in the journal Energy Material Advances.
“Fluid optional batteries hold significant commitment for fixed energy capacity,” notes lead scientist Teacher Ying Bai, subsidiary with the School of Materials Science and Designing at the Beijing Establishment of Innovation.
“While business lithium-particle batteries have seen far and wide reception in transportation and different adaptable gadgets throughout the past many years, their true capacity as energy stockpiling sources is on the ascent. By and by, the restricted accessibility of lithium and concerns in regards to the security of natural electrolytes highlight the need for a continuous mission for options more qualified to fixed energy capacity.”
“Aqueous zinc-based batteries have piqued the interest of researchers due to zinc metal’s favorable redox potential and excellent specific capacity. However, achieving good performance in practical applications requires more efforts.”
Lead researcher Professor Ying Bai, affiliated with the School of Materials Science and Engineering at Beijing Institute of Technology.
Bai made sense of the fact that watery optional batteries certainly stand out as the planned and practical contender for framework-level energy stockpiling due to the safe fluid electrolytes lately.
“Fluid zinc-based batteries definitely stand out from analysts because of the good redox potential and the great explicit limit of zinc metal. Nevertheless, accomplishing agreeable execution in down-to-earth applications actually requires further undertakings,” expressed Bai.
“Among the different parts of the battery, the cathode material assumes an urgent role in deciding the electrochemical qualities of fluid zinc-based batteries. Until now, a few classes of terminals have been utilized as cathodes for fluid zinc-based batteries, for example, Prussian blue analogs and manganese-based oxides.”
“In any case, the restricted explicit limit of PBAs compels their more extensive application in fluid zinc-based batteries. Moreover, manganese oxides and vanadium oxides have found far-reaching use in watery zinc-based batteries, at first conveying high limits going from 300 mAh g-1 to 500 mAh g-1. Be that as it may, because of cathode disproportionation responses and host structure stage changes, the cycling life expectancy remains lacking for the reasonable sending of fluid zinc-based batteries.”
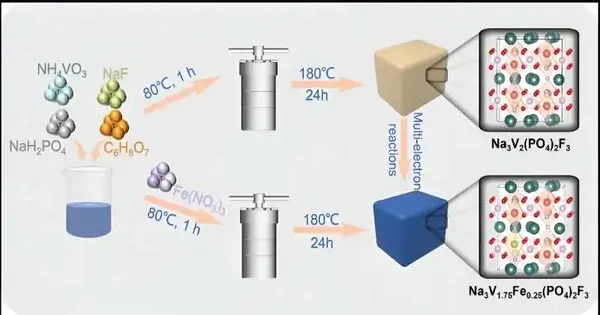
As indicated by Bai, sodium superionic guides (NASICONs) are considered cutthroat cathode materials for fluid optional batteries since they have a hearty polyanionic system with an overall equation of AxM1M2(XO4)3 (A = Li, Na; M1 and M2 = V, Fe, Mn, Ti, Cr; X = P, S).
“The three-layered open system structure not only forms a dissemination pathway for metal particles but additionally restricts the grid volume change in the intercalation response, which brings about great rate execution and cycle life expectancy,” Bai said. “The covalent holding obtained from the polyanionic structure areas of strength for and impacts hypothetically take into consideration higher working voltage, which adds to the energy thickness of the NASICONs cathode.”
“Notwithstanding the gigantic advancement that has been accomplished, ordinary NASICONs materials in fluid electrolytes commonly have low working voltages, low energy densities, and an unfortunate life expectancy, making their applications troublesome.”
As per Bai, a normal NASICON compound, Na3V2(PO4)2F3 (NVPF), has been arranged as the cathode for Zn2+ stockpiling, yet its further application is tormented by the primary decay and low down-to-earth limit due to serious areas of strength for the Zn2+ with the host material.
“The use of multi-particle electrolytes is, by all accounts, quite possibly the best procedure, where the cathode material is settled by the synergistic association of various cations,” Bai said.
“For sure, NVPF could be used as a cathode in the watery zinc/sodium batteries with a zinc metal anode, where Na+ or Zn2+ is embedded or separated into or from the NVPF cathode, and Zn2+ is saved or broken up onto or from the anode. Be that as it may, the change in metal disintegration during Na+ or Zn2+ intercalation or extraction actually happens in the fluid zinc/sodium batteries, actuating the unavoidable limit rot.”
Progress in metal particle replacement addresses a promising system to upgrade underlying soundness and expand the high expected limit,” underscored Bai. “Given the relevant expense considerations and expected monetary benefits, our examination group attests that outfitting minimal-cost, plentifully accessible change metal substitutes is basic for the progression of watery zinc/sodium batteries, including NASICON cathodes.”
More information: Jiasheng Yue et al, Initiating High-Voltage Multielectron Reactions in NASICON Cathodes for Aqueous Zinc/Sodium Batteries, Energy Material Advances (2023). DOI: 10.34133/energymatadv.0050