Specialists who are attempting to find options in contrast to lithium-particle batteries stand out enough to be noticed by potassium-particle batteries. Potassium is a plentiful asset, and its innovation capabilities are similar to those of lithium-particle batteries; however, these batteries have not been created at a huge scale on the grounds that the ionic range creates some issues in energy capacity and unacceptable electrochemical execution.
To take care of this issue, specialists are thinking about using NiCo2Se4, a bimetallic selenide, to make circle-molded terminals. The circles are developed with NiCo2Se4 nanotubes, which work on the electrochemical reactivity for quicker movement and capacity of potassium particles.
The exploration was introduced in a paper distributed in Energy Materials and Gadgets on September 14.
“Bimetallic selenides join the enhancing elements of two metals, which synergize by showing rich redox response locales and high electrochemical exercises. One bimetallic selenide, NiCo2Se4, was recently read up for sodium capacity, supercapacitors, and electrocatalysts and presents impressive potential for potassium particle capacity.
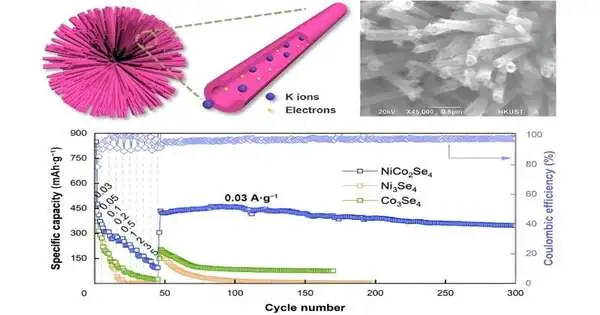
“In terms of cyclic stability and rate capability, the NiCo2Se4 nanotube electrode performed significantly better than other tested electrodes, including Ni3Se4 and Co3Se4. This is due to NiCo2Se4’s unique nanotube structure and the synergy provided by the presence of two metals.”
Mingyue Wang, a researcher at the Engineering Research Center of Energy Storage Materials and Devices.
“By blending NiCo2Se4 utilizing a two-step aqueous cycle, a nanotube structure with blossom-like groups is created, making helpful channels for potassium particle and electron movement,” said Mingyue Wang, a scientist at the Designing Exploration Focus of Energy Stockpiling Materials and Gadgets at Xi’an Jiaotong College in Xi’an, China.
At first, Ni-Co forerunner circles with strong nanoneedles are ready. These circles have an obvious translucent design that is then presented to selenide during a cycle called selenization. This cycle acquaints selenium with the Ni-Co forerunner, fostering the NiCo2Se4 nanotube shell.
The empty cylinders have a structure in view of a peculiarity called the Kirkendall impact, which is when two metals move due to the distinction in the dissemination paces of their iotas. These nanotubes are around 35 nanometers wide, giving adequate room for the potassium particles and electrons to move.
Through various tests and examinations, the specialists had the option to affirm how well the NiCo2Se4 anodes could move and store potassium particles and electrons. They found that NiCo2Se4 has more dynamic locales than other anode materials, has consistently dispersed components, and beats different terminals that were tried during research.
“The NiCo2Se4 nanotube cathode introduced a vastly improved electrochemical execution with regards to cyclic security and rate capacity compared to other tried terminals, including Ni3Se4 and Co3Se4. This is on the grounds of the interesting nanotube design of NiCo2Se4 and the collaboration presented by the co-presence of two metals,” said Wang.
These monometallic partners, Ni3Se4 and Co3Se4, were not generally as fruitful as the bimetallic NiCo2Se4, essentially on account of the way the two metals (Ni and Co) interfaced together. NiCo2Se4 likewise had a higher limit, which is extremely useful for keeping up with cyclic strength and high-rate execution.
“This work offers new experiences into the plan of miniature/nano-organized double metal selenides as anodes for potassium-particle batteries with unprecedented potassium particle capacity execution,” said Wang.
More information: Mingyue Wang et al, Conversion mechanism of NiCo 2Se 4 nanotube sphere anodes for potassium-ion batteries, Energy Materials and Devices (2023). DOI: 10.26599/EMD.2023.9370001