Since the 1970s, researchers have realized that copper has an exceptional capacity to change carbon dioxide into significant synthetic compounds and materials. Yet, for a long time, researchers have attempted to comprehend how this normal metal functions as an electrocatalyst, an instrument that utilizes energy from electrons to change particles into various items synthetically.
Presently, an examination group led by Lawrence Berkeley Public Lab (Berkeley Lab) has acquired new understanding by catching constant films of copper nanoparticles (copper particles designed at the size of a billionth of a meter) as they convert CO2 and water into inexhaustible energies and synthetic substances: ethylene, ethanol, and propanol, among others. Last week, the work was recorded in Nature’s diary.
“This is extremely invigorating.” “After a long time of work, we’re finally ready to show, with clear evidence, how copper electrocatalysts succeed in CO2 reduction,” said Peidong Yang, a senior staff researcher in Berkeley Lab’s Materials Sciences and Synthetic Sciences Divisions who led the review. Yang is likewise a teacher of science and materials science and design at UC Berkeley.
“This is quite fantastic. We are finally able to demonstrate, with incontrovertible proof, how copper electrocatalysts excel at CO2 reduction after decades of work.”
Peidong Yang, a senior faculty scientist in Berkeley Lab’s Materials Sciences and Chemical Sciences Divisions.
“Realizing how copper is such a brilliant electrocatalyst brings us steps nearer to transforming CO2 into new, inexhaustible, sun-based fuels through fake photosynthesis.”
The work was made conceivable by combining another imaging method called operando 4D electrochemical fluid cell STEM (checking transmission electron microscopy) with a delicate X-beam test to explore a similar example: copper nanoparticles in fluid. The first creator, Yao Yang, a UC Berkeley mill operator postdoctoral individual, imagined the notable methodology under the direction of Peidong Yang while making progress toward his Ph.D. in science at Cornell College.
Researchers who concentrate on counterfeit photosynthesis materials and responses have needed to combine the force of an electron test with X-beams, yet the two methods ordinarily can’t be performed by a similar instrument.
Electron magnifying lenses (like STEM or TEM) use light emissions and succeed at portraying the nuclear structure in pieces of material. 4D STEM (or “2D raster of 2D diffraction designs utilizing examination transmission electron microscopy”) instruments, for example, those at Berkeley Lab’s Sub-atomic Foundry, have recently pushed the limits of electron microscopy significantly further, enabling researchers to delineate nuclear or atomic locales in various materials, ranging from hard metallic glass to delicate, adaptable films.
Then again, delicate (or lower-energy) X-beams are valuable for distinguishing and following substance responses continuously in an operando, or genuine world, climate.
Yet, presently, researchers cannot have the most ideal scenario. At the core of the new strategy is an electrochemical “fluid cell” test holder with amazing flexibility. Multiple times more slender than a human hair, the gadget is viable with both STEM and X-beam instruments.
The electrochemical fluid cell’s ultrathin configuration permits solid imaging of fragile examples while shielding them from electron beam harm. A unique terminal specially crafted by co-creator Cheng Wang, a staff researcher at Berkeley Lab’s High Level Light Source, empowered the group to direct an X-beam and explore different avenues regarding the electrochemical fluid cell. Joining the two permits specialists to extensively describe electrochemical responses both progressively and at the nanoscale.
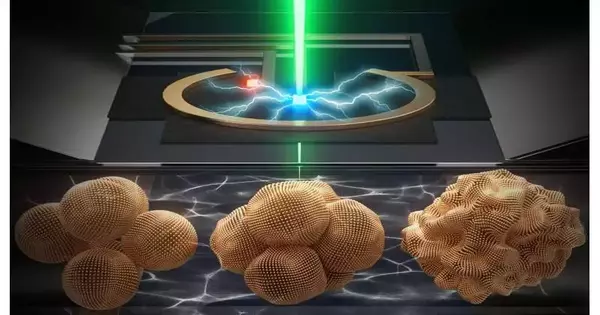
Video of a 4D-STEM try during which Yao Yang and group utilized the new electrochemical fluid cell to notice copper nanoparticles (ranging in size from 7 nanometers to 18 nanometers) develop into dynamic nanograins during CO2 electrolysis—a cycle that utilizes power to drive a response on the outer layer of an electrocatalyst. The new electrochemical fluid cell permits scientists to determine the images of items smaller than 10 nanometers.
During 4D-STEM tests, Yao Yang and her group utilized the new electrochemical fluid cell to notice copper nanoparticles (ranging in size from 7 nanometers to 18 nanometers) develop into dynamic nanograins during CO2 electrolysis—aa cycle that utilizes power to drive a response on the outer layer of an electrocatalyst.
The tests uncovered a shock: copper nanoparticles consolidated into bigger metallic copper “nanograins” not long after the electrochemical response.
To find out more, the group went to Wang, who spearheaded a procedure known as “thunderous delicate X-beam dispersing (RSoXS) for delicate materials” at the high-level light source over a long time ago.
With Wang’s assistance, the research team used a similar electrochemical fluid cell, but this time during RSoXS tests, to determine whether copper nanograins work with CO2 reduction.Wang realized that delicate X-beams are ideal for studying how copper electrocatalysts progress during CO2 reduction. By utilizing RSoXS, specialists can continuously screen for various responses among a large number of nanoparticles and precisely recognize substance reactants and items.
The RSoXS tests at the high-level light source, alongside extra proof accumulated at the Cornell High Energy Synchrotron Source (CHESS), demonstrated that metallic copper nanograins act as dynamic locales for CO2 decrease. (Metallic copper, otherwise called copper (0), is a type of the component copper.)
During CO2 electrolysis, the copper nanoparticles change their design during an interaction called “electrochemical scrambling.” The copper nanoparticles’ surface layer of oxide corrupts, making open locales on the copper surface for CO2 particles to join, which made sense to Peidong Yang. Furthermore, as CO2 “docks” or ties to the copper nanograin surface, electrons are then moved to CO2, causing a response that all the while produces ethylene, ethanol, and propanol alongside other multicarbon items.
“The copper nanograins basically transform into minimal substance-producing manufacturing plants,” Yao Yang said.
Further investigations at the sub-atomic foundry, the high-level light source, and CHESS uncovered that size matters. All of the 7-nanometer copper nanoparticles participated in the CO2 decrease, though the bigger nanoparticles didn’t. Furthermore, the researchers discovered that primary metallic copper can effectively reduce CO2 in multicarbon products. The discoveries have suggestions for “soundly planning productive CO2 electrocatalysts,” Peidong Yang said.
The new concentrate also confirmed Peidong Yang’s 2017 discoveries: that the 7-nanometer-sized copper nanoparticles require low energy contributions to begin CO2 reduction. As an electrocatalyst, the 7-nanometer copper nanoparticles required a record-low main thrust of around 300 millivolts, which is not exactly commonplace for mass-produced copper electrocatalysts. The best-performing impetuses that produce multicarbon items from CO2 regularly work at a high main thrust of 1 volt.
Copper nanoparticles may actually improve the energy effectiveness and efficiency of certain impetuses intended for forgery photosynthesis, a field of study that aims to create sun-oriented fills from daylight, water, and CO2.Analysts within the Department of Energy-supported Fluid Daylight Union (LiSA) are currently planning to use copper nanograin impetuses in the design of future solar-powered fuel gadgets.
“The procedure’s capacity to record constant films of a substance interaction opens up thrilling chances to concentrate on numerous other electrochemical energy change processes.” “It’s a gigantic forward leap, and it could never have been conceivable without Yao and his spearheading work,” Peidong Yang said.
More information: Yao Yang et al, Operando studies reveal active Cu nanograins for CO2 electroreduction, Nature (2023). DOI: 10.1038/s41586-022-05540-0