Empty, organized, upheld metal impetuses (for example, nanoreactor impetuses) with embodied dynamic destinations and obvious shells give an optimal spot to multicomponents to respond or change helpfully in a methodical way, and proficiently have been perceived as one of the most well-known impetus applicants.
Despite the fact that reactant improvement has been proposed by exploring the connection between synergist execution and the construction of nanoreactors at the nanolevel, the investigation of the enhancement impact at the mesoscale (500–2000 nm) is not yet thorough enough. Building the nanoreactor models with dynamic metals inside and outside the empty nanostructure by means of various engineered strategies or successions will unavoidably influence the microenvironment around the dynamic locales as well as the fundamental dynamic destinations.
Moreover, reactant enhancement at the mesoscale level includes many cycles, for example, adsorption and dispersion, which can’t be expounded by building straightforward computational models at the nanoscale level. In this way, examination of the reactant advancement at the mesoscale level requires keeping up with the characteristic dynamic locales while developing the exploration model, regardless of enhancement conduct.
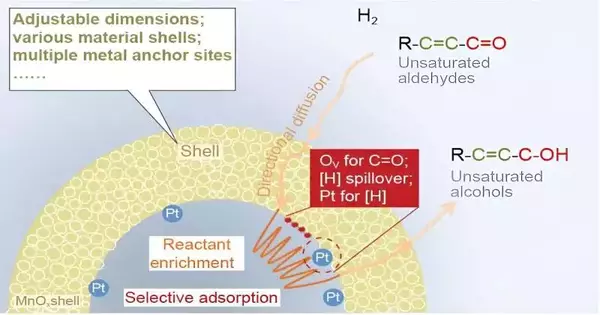
In another exploration article distributed in the Public Science Survey, researchers at the Dalian Foundation of Synthetic Physical Science (DICP), the College of Chinese Institute of Sciences, Taiyuan College of Innovation, the College of Surrey, and Inward Mongolia College present a new nanoreactor impetus (Pt NPs@MnOx) with consistently scattered Pt nanoparticles exemplified in an oxygen opportunity-rich MnOx empty design to catalyze the specific hydrogenation of CAL and examine reactant improvement at the mesoscale level.
The synergist execution for CAL-specific hydrogenation on Pt NPs@MnOx is 3.4-fold higher than that of Pt NPs&MnOx, which is truly squashed into an open construction. UV-vis, in situ FTIR, and IGA estimations exhibit that the empty MnOx shell of Pt NPs@MnOx prompts higher CAL take-up.
The component behind this peculiarity might comprise two stages. As the empty construction makes a bound space, external reactants would ceaselessly diffuse into the inside of the empty design, directionally driven by the fixation slope as well as slim can imagine impact (stage 1).
Then, at that point, these reactants are fixed on the internal surface by adsorption to keep the nearby low focus in the bound space. Conversely, Pt NPs and MnOx couldn’t uphold this directional dissemination process. Additionally, DFT results uncover that CAL is all the more emphatically adsorbed on the outer layer of Pt NPs@MnOx than Pt NPs&MnOx under overabundance reactants (stage 2).
H2-TPR-MS and limited component recreation results likewise show that the Pt NPs@MnOx nanoreactor makes a steady space with a high fixation and low stream rate to forestall the breakage of the reactants (separated hydrogen). It is in this manner clear that reactant improvement is gotten from the directional dispersion of reactant passed through a nearby fixation slope and an expanded measure of reactant adsorbed because of the upgraded adsorption capacity in empty MnOx.
The Pt NPs@MnOx impetus displays very high synergist exercises and selectivity in an extensive variety of response pressures. A 95% transformation with 95% COL selectivity is gotten on Pt NPs@MnOx at just 0.5 MPa H2 and 40 min, which is a somewhat gentle condition contrasted with the most detailed synergist frameworks.
Joining exploratory outcomes with thickness practical hypothesis computations, the unrivaled cinnamyl liquor (COL) selectivity starts with the particular adsorption of CAL and the fast arrangement and desorption of COL in the MnOx shell. Besides, the empty void initiates the reactant improvement process, upgrading the response action.
These discoveries offer the chance of upgrading the reactant execution at the mesoscale level by planning a judicious nanoreactor, as opposed to lessening the size of the metal particles or changing them with heteroatoms or ligands at the nanoscale level.
More information: Yanfu Ma et al. Reactant enrichment in hollow void of Pt NPs@MnOx nanoreactors for boosting hydrogenation performance, National Science Review (2023). DOI: 10.1093/nsr/nwad201