Solar-powered microbes are microorganisms that can use sunlight as an energy source for photosynthesis, such as cyanobacteria or algae. Using the energy from sunlight, these microbes absorb carbon dioxide from their surroundings and convert it into organic molecules such as sugars or starches.
Researchers created a multimodal platform for imaging biohybrids, which are microorganisms that use solar energy to convert carbon dioxide into value-added chemical products, in order to better understand how they work and how they can be optimized for more efficient energy conversion.
Bacteria may not be the first thing that comes to mind when considering ways to generate environmentally friendly products in a sustainable manner.
However, scientists have recently developed microbe-semiconductor biohybrids, which combine the biosynthetic power of living systems with the ability of semiconductors to harvest light. Solar energy is used by these microorganisms to convert carbon dioxide into value-added chemical products such as bioplastics and biofuels. However, it is unclear how that energy transport occurs in such a small, complex system, and whether the process can be improved.
Biology is extremely diverse. Individual cells are extremely diverse. Now, in order to better interrogate it, you really need to measure it at the single-cell level. This is where we come into play.
Peng Chen
Cornell University researchers have developed a multimodal platform to image these biohybrids with single-cell resolution, to better understand how they function and how they can be optimized for more efficient energy conversion.
The team’s paper, “Single-Cell Multimodal Imaging Uncovers Energy Conversion Pathways in Biohybrids,” published in Nature Chemistry. The co-lead authors are postdoctoral researcher Bing Fu and former postdoctoral researcher Xianwen Mao.
The project was led by Peng Chen, professor of chemistry in the College of Arts and Sciences. The effort is an offshoot of a larger collaboration — with Tobias Hanrath, professor at the Smith School of Chemical and Biomolecular Engineering in Cornell Engineering, and Buz Barstow, assistant professor of biological and environmental engineering in the College of Agriculture and Life Sciences — that was funded by the U.S. Department of Energy (DOE) to explore microscopic imaging of microbes as a way to advance bioenergy research.
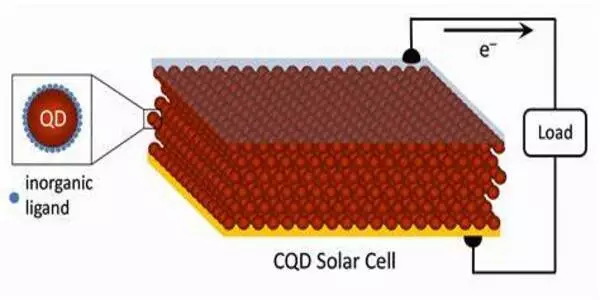
Biohybrid research has typically been carried out with bacteria in bulk – essentially a large number of cells in a bucket, according to Peng – with an emphasis on the overall yield of the value-added chemicals and the collective behaviors of the cells, rather than the underlying mechanism that enables the complex chemical transformation.
“Biology is extremely diverse. Individual cells are extremely diverse. Now, in order to better interrogate it, you really need to measure it at the single-cell level,” Chen explained. “This is where we come into play. We offer quantitative assessments of protein behaviors as well as a mechanistic understanding of how electron transport from the semiconductor to the bacteria cell occurs.”
To survey the bacterium Ralstonia eutropha, the new platform combined multi-channel fluorescence imaging with photoelectrochemical current mapping. The platform was able to image, track, and quantify multiple proteins in the cell while also measuring electron flow, eventually correlating cellular protein properties and electron transport processes.
The researchers were able to distinguish the functional roles of two types of hydrogenases that help metabolize hydrogen and drive CO2 fixation: one bound to the cell membrane and one soluble in the cytoplasm. While soluble hydrogenase is known to be essential for hydrogen metabolism, the researchers discovered that membrane-bound hydrogenase, while less important, actually facilitates and improves the process.
In addition, the researchers obtained the first experimental evidence that the bacteriacan uptake a large amount of electrons from semiconductor photocatalysts. The team measured the electron current and found it be three orders of magnitude larger than what scientists previously thought, which suggests that future bacteria strains could be engineered to improve the efficiency of energy conversion.
Additionally, the researchers discovered that membrane-bound and soluble hydrogenases play an important role in mediating electron transport from the semiconductor into the cell. Meanwhile, the cell can not only accept electrons but also spit them out in the opposite direction, without the help of hydrogenases.
The imaging platform is sufficiently generalizable to be applied to the study of other biological-inorganic systems, including yeast, as well as other processes such as nitrogen fixation and pollutant removal.