Immune system issues are among the most pervasive ongoing illnesses across the globe. Arising medicines for immune system problems center around “receptive cell treatments,” or those utilizing cells from a patient’s own body to accomplish immunosuppression. These helpful cells are perceived by the patient’s body as “self,” subsequently restricting aftereffects, and are explicitly designed to confine the planned remedial impact.
In treating immune system illnesses, current supportive cell treatments have generally revolved around the administrative lymphocyte (Treg), which is characterized by the outflow of the Forkhead box protein 3, or Foxp3. In spite of the fact that Tregs offer extraordinary potential, involving them for helpful purposes remains a significant test. Specifically, current conveyance strategies bring about the wasteful design of immune system microorganisms.
Tregs just make roughly 5%–10% out of flowing fringe blood mononuclear cells. Besides, Tregs need more unambiguous surface markers that separate them from other lymphocyte populations. These obstacles make it hard to gather, filter, and develop Tregs to remedially pertinent numbers. In spite of the fact that there are extra tissue-occupant Tregs in non-lymphoid organs, for example, in skeletal muscle and instinctive fat tissue, these Tregs are seriously distant and low in number.
“These modified T cells can decrease effector T cell function, which is essential because T cell hyperactivity is a prevalent characteristic in autoimmune disorders. The mRNA encodes for Foxp3 protein, which is a transcription factor that renders the T cells immunosuppressive rather than aggressive.”
Explains first author Ajay Thatte, a doctoral student and NSF Fellow in the Mitchell Lab.
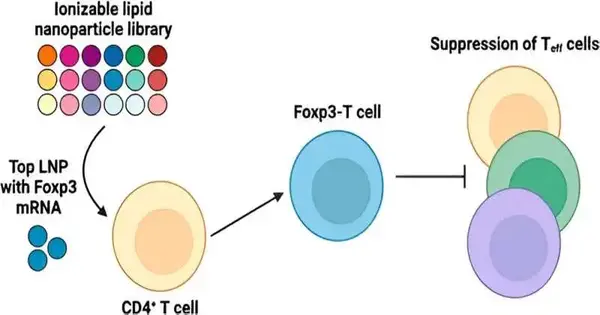
Presently, an exploration group led by Michael Mitchell, academic administrator in bioengineering in the School of Designing and Applied Science at the College of Pennsylvania, has fostered a lipid nanoparticle (LNP) stage to convey Foxp3 courier RNA (mRNA) to white blood cells for applications in autoimmunity. Their discoveries are distributed in the Nano Letters diary.

Passed on to right: Ajay Thatte, Benjamin Nachod, Rohan Palanki, Kelsey Swingle, Alex Hamilton, and Michael Mitchell. Credit: Mitchell Lab
“The significant difficulties related to ex vivo (outside the body) cell design are effectiveness, poisonousness, and scale-up; our mRNA lipid nanoparticles (mRNA LNPs) permit us to beat these issues,” says Mitchell. “Our work’s oddity comes from three significant parts: first, the utilization of mRNA, which considers the age of transient immunosuppressive cells; second, the utilization of LNPs, which take into account compelling conveyance of mRNA and productive cell design; and last, the ex vivo design of essential human lymphocytes for immune system infections, offering the most immediate pipeline for clinical interpretation of this treatment from seat to bedside.”
“As far as anyone is concerned, this is one of the primary mRNA LNP stages that has been utilized to design white blood cells for immune system treatments,” he proceeds. “Extensively, this stage can be utilized to design receptive cell treatments for explicit immune system infections and might possibly be utilized to make remedial roads for sensitivities, organ transplantation, and then some.”
Conveying the Foxp3 protein to lymphocytes has been troublesome in light of the fact that proteins don’t promptly cross the cell film. “The mRNA encodes for Foxp3 protein, which is a record factor that makes lymphocytes immunosuppressive as opposed to dynamic,” makes sense of first creator Ajay Thatte, a doctoral understudy and NSF individual in the Mitchell Lab. “These designed immune system microorganisms can smother lymphocyte capability, which is significant as white blood cell hyperactivity is a typical aggregate in immune system illnesses.”
Additionally, the size, charge, and moderately low intricacy of mRNA permit it to be handily bundled into compelling conveyance frameworks, for example, ionizable LNPs, which have been shown to be a strong conveyance stage, specifically for mRNA, as exhibited by the outcome of the coronavirus immunizations.
In the new review, Mitchell and his group originally screened a library of 18 one-of-a-kind LNPs to distinguish a top-performing LNP for mRNA conveyance to human CD4+ lymphocytes. Then, at that point, they re-figured out this LNP for the Foxp3 mRNA to produce Foxp3-lymphocytes, making sure that the cells actually stifled effector immune system microorganism multiplication. The outcomes exhibit the capability of involving mRNA LNPs to design immunosuppressive cell-based treatments for immune system infections, and that’s just the beginning.
In later examinations, the group intends to analyze the suppressive impact of these designed Foxp3-lymphocytes on other resistant cells like macrophages and dendritic cells. They then, at that point, desire to move the designed Foxp3-white blood cells into mouse models of immune system infection to test their adequacy and immunological impact. At long last, they’ll hope to create designated LNPs to convey Foxp3 mRNA to white blood cells coursing in the body, laying out an in-situ design stage for immune system illness treatments.
“Albeit broadly investigated in malignant growth applications, designing safe cells outside the body has been less investigated for autoimmunity applications. Also, achieving high productivity and low poisonousness during the phone design cycle is very troublesome,” says Mitchell. “Our LNP innovation considers the simple and proficient design of immune system microorganisms with low poisonousness. Our designed white blood cells can be utilized across a scope of hyperactive, safe issues.”
More information: Ajay S. Thatte et al, mRNA Lipid Nanoparticles for Ex Vivo Engineering of Immunosuppressive T Cells for Autoimmunity Therapies, Nano Letters (2023). DOI: 10.1021/acs.nanolett.3c02573