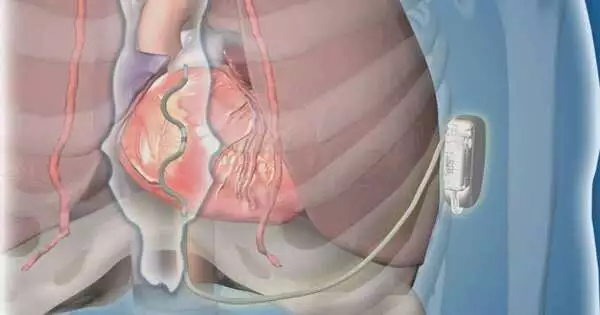
Another sort of extravascular implantable cardioverter-defibrillator (ICD) utilizing a lead (slim wire) set behind the sternum met security and viability objectives for members in a premarket worldwide clinical review. The device really ended intense and ongoing perilous ventricular arrhythmias. The discoveries were introduced during a late-breaking meeting at the European Society of Cardiology Congress and were published in The New England Journal of Medicine.
For example, ventricular fibrillation and ventricular tachycardia occur in the lower heart chambers, or ventricles. They are risky on the grounds that they impede the typical composed filling and siphoning of blood through the heart. If not quickly treated, these arrhythmias can cause breakdown and demise. The capacity of ICDs to precisely identify and end ventricular arrhythmias in high-endanger patients saves lives.
For concentrating on members, the lead of the extravascular ICD was embedded under the sternum, contrasting with transvenous ICD drives that are embedded through the veins into the heart or subcutaneous ICDs that have a lead put underneath the skin over the sternum. Patients with earlier open-heart medical procedures, or who required pacing for a sluggish pulse or had a pacemaker, were not candidates for this review.
“The novel extravascular ICD supplied anti-tachycardia pacing—rapid pacing—to painlessly terminate 70% of the ventricular tachycardia episodes for which it was used, (which is) at least as excellent as transvenous ICDs and not accessible in the subcutaneous ICD,”
Dr. Friedman
This study is empowering. By setting the lead in this new situation behind the sternum, the remarkable yet serious dangers related to transvenous ICDs, for example, lung breakdown, harm to heart valves, and heart hole, can be kept away from. The subcutaneous ICD’s limits are also preserved.Since the lead is behind the sternum and near the heart, pacing can be conveyed, and defibrillation requires less energy with a more extended battery duration than with the subcutaneous ICD, says Paul Friedman, M.D., a cardiovascular electrophysiologist, and head worldwide examiner and first creator of the review. Dr. Friedman chairs the Department of Cardiology at the Mayo Clinic in Rochester.
“The new extravascular ICD communicated the enemy of tachycardia pacing—fast pacing—to easily end 70% of the ventricular tachycardia episodes for which it was applied, (which is) as great as transvenous ICDs and not accessible in the subcutaneous ICD,” Dr. Friedman says.”The extravascular ICD was also capable of providing reinforcement pacing to prevent stops and had the option to truly defibrillate utilizing a device a fraction of the size of the subcutaneous ICD.”
Analysts from 17 nations took part in the review. Of the 316 patients with an endeavored embed, 299 were released with a working extravascular ICD framework. The defibrillation achievement rate was 98.7%. At a half year, 92.6% of members had no significant framework or system-related confusion.
“It is vital to take note of that electrophysiologists don’t regularly put gadget leads behind the sternum. Nonetheless, we found that with a hearty preparation program, we could securely do as such in the electrophysiology lab. Starting implantations at each site incorporated a cardiologist and a heart specialist. The typical method time was 74 minutes, like that of the early subcutaneous ICD experience, “says Yong-Mei Cha, M.D., head of the Implantable Device Lab at Mayo Clinic and site head agent of the review.
“While empowering, these outcomes mirror an early encounter, and there is something else to be learned with longer development and more prominent use,” says Dr. Friedman. “The rates of unexpected shock are higher than with flow gadgets, but comparable or lower than the early involvement in other types of defibrillators, and steps have previously been taken to reduce them.” Also, this new gadget isn’t ideal for everybody. Patients with past open-heart medical procedures were barred from the review and wouldn’t be contenders for this therapy as of now.
More information: Paul Friedman et al, Efficacy and Safety of an Extravascular Implantable Cardioverter–Defibrillator, The New England Journal of Medicine (2022). DOI: 10.1056/NEJMoa2206485
Journal information: New England Journal of Medicine