Nitric oxide production in the brain is linked to autistic symptoms, according to Israeli researchers at the University of Haifa and the Hebrew University of Jerusalem. The researchers describe how an initial observation in a mouse study led to a deeper investigation into the activity of nitric oxide and the discovery of a quantity-dependent causative correlation to disease pathology in their paper, “The NO Answer for Autism Spectrum Disorder,” which was published in Advanced Science.
The review results show that nitric oxide (NO) plays a part in mental imbalance range jumble (ASD) improvement and influences synaptogenesis and the glutamatergic and GABAergic frameworks in the cortex and the striatum, uniting into ASD-like social shortages. Animal models, cortical neurons derived from human iPSCs, and clinical samples all support the experiments’ finding of a direct link between NO and ASD.
A neurodevelopmental and behavioral disorder known as autism spectrum disorder typically begins in infancy. It is portrayed by irregularities in friendly collaborations, shortages in correspondence, limited interests, and a monotonous way of behaving, influencing 1 in every 44 kids worldwide.
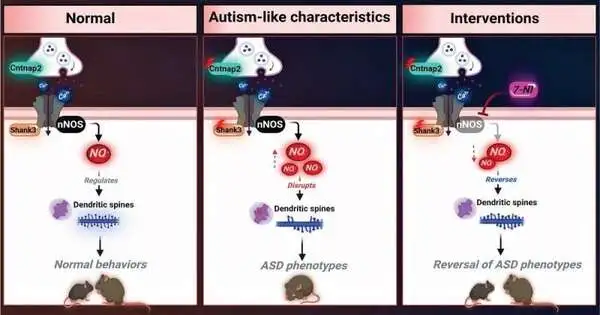
ASD has been linked to specific gene pathologies. Cancellations or changes in the SHANK3 quality and CNTNAP2 quality with loss-of-capability transformations are exceptionally embroiled. It suggests that a mechanism related to how those genes are meant to function normally has been disrupted when researchers discover genetic correlations with a disease. Finding the molecular cause of these disruptions can be more difficult.
The researchers have previously found elevated levels of nitric oxide in mice that have been genetically altered to mimic ASD. NO is a multifunctional flagging particle and synapse that manages cell endurance, separation, and expansion of neurons, as well as synaptic movement, pliancy, and vesicle dealing.
Mice were given S-nitroso-N-acetyl penicillamine for ten days to see if it could cause phenotypes similar to ASD. Similar to the genetically altered ASD mice, this increased NO production led to a significant decrease in the mice’s cortical dendritic spine density.
The researchers then tried the experiment in the opposite direction by inhibiting NO formation in ASD-modified mice. Treatment increased the number of dendritic spines in this group, possibly reversing some ASD effects caused by genetics.
Although these biochemical results are encouraging, the mice’s behavioral phenotypes may not necessarily change. So the scientists reproduced the trial conditions and put altered mice through tests.
Normal mice spent significantly more time exploring the novel object than the familiar object, according to a novel object recognition (NOR) test. In contrast, the mice with ASD did not prefer unfamiliar objects over novel ones. This suggests that the ASD bunch had deficiencies in oddity chasing and memory. ASD-modified mice significantly preferred the novel object to the familiar object after being treated with a NO inhibitor.
It is significant that the study found a mechanistic link between nitric oxide levels and autism spectrum disorder. It is the kind of significant discovery that is typically followed by a word salad of caveats and suggestions that it may open the way for subsequent research to discover a key to unlock the door of potential clinical treatments.
In this instance, the researchers may have gone straight to that door with the key in hand in search of a clinical treatment target by demonstrating a reversal of pathology by specifically targeting a single mechanism while testing a hypothesis.
More information: Manish Kumar Tripathi et al, The NO Answer for Autism Spectrum Disorder, Advanced Science (2023). DOI: 10.1002/advs.202205783