In 1842, the popular English specialist Michael Faraday mentioned an astounding observable fact by some coincidence: A flimsy layer of water structures on the outer layer of ice, despite the fact that it is well below zero degrees. The temperature is below the liquefying point of ice, yet the outer layer of the ice has dissolved. This fluid layer on the ice beneath the precious stones is additionally why snowballs stay together.
It was only around 140 years after the fact, in 1985, that this “surface softening” could be logically affirmed under controlled lab conditions. Surface softening has been shown in different translucent materials and is experimentally known as follows: A few degrees below the genuine liquefying point, a fluid layer a couple of nanometers thick forms on the outer layer of the generally strong material.
Since the surface properties of materials assume an urgent part in their utilization as, for example, impetuses, sensors, battery anodes, and then some, surface dissolving isn’t just of key significance yet in addition considering specialized applications.
It should be emphasized that this interaction has literally nothing to do with the impact of, say, removing a 3D ice cube from the cooler and presenting it at room temperature. The justification for why an ice shape dissolves on its surface first under such circumstances is that the surface is fundamentally hotter than the ice block’s inside.
Surface softening is distinguished in glass
In precious stones with occasionally organized molecules, the dainty fluid layer on a superficial level is normally recognized by dispersing tests, which are extremely delicate in the presence of nuclear request. Since fluids are not organized in a normal example, such procedures can obviously determine the presence of a thin fluid film on top of a thick one.
This methodology, nonetheless, doesn’t work for glasses (for example, cluttered, formless materials) since there is no distinction in the nuclear request between the strong and the fluid. In this way, the surface liquefaction of glasses has remained somewhat neglected in testing.
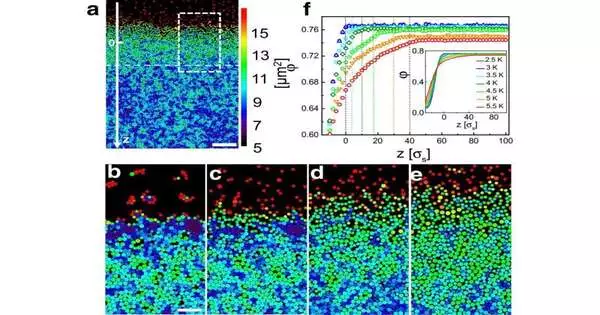
To conquer the previously mentioned challenges, Clemens Bechinger, physical science teacher at the College of Konstanz, and his partner Li Tian utilized a stunt: rather than concentrating on a nuclear glass, they created a disorganized material made of tiny glass circles known as colloids. Rather than molecules, these particles are multiple times bigger and can be noticed straightforwardly under a magnifying instrument.
The specialists had the option of showing the course of surface dissolving in such a colloidal glass on the grounds that the particles close to the surface move a lot quicker compared with the strong particles beneath. From the get-go, such a way of behaving isn’t altogether startling, since the molecule thickness at the surface is lower than in the hidden mass material. Consequently, particles near the surface have more space to move past one another, which makes them quicker.
An astounding disclosure
What astonished Clemens Bechinger and Li Tian, nonetheless, was the way that even far beneath the surface, where the molecule thickness has arrived at the mass worth, the molecule versatility is still altogether higher compared with the mass material.
The magnifying lens pictures show that this beforehand obscure layer depends on 30 molecule widths and goes on from the surface into the more profound districts of the strong in a streak-like example. “This layer, which ventures far into the material, has intriguing material properties since it consolidates fluid and strong elements,” Bechinger makes sense of.
As an outcome, the properties of dainty, disarranged films rely especially upon their thickness. As a matter of fact, this property is now being taken advantage of in their utilization as slight ionic guides in batteries, which are found to have a fundamentally higher ionic conductivity compared with thick films. With the new experiences acquired from the examinations, be that as it may, this conduct can now be seen quantitatively and consequently be improved for specialized applications.
The exploration was distributed in Nature Correspondences.
More information: Li Tian et al, Surface melting of a colloidal glass, Nature Communications (2022). DOI: 10.1038/s41467-022-34317-2