As the world’s hunger for carbon-based materials like graphite expands, Ohio University specialists introduced proof this week for another carbon material they named “undefined graphite.”
Physicist David Drabold and engineer Jason Trembly began with the inquiry, “Might we at any point make graphite from coal?”
“Graphite is a significant carbon material with many purposes.” A prospering application for graphite is for battery anodes in lithium-particle batteries, and it is urgent for the electric vehicle industry—a Tesla Model S on normal requirements has 54 kg of graphite. Such anodes are ideal whenever made with unadulterated carbon materials, which are turning out to be harder to get attributable to spiraling mechanical interest, “they write in their paper, “Stomach muscle initio recreation of undefined graphite,” which was distributed today in Physical Review Letters.
“We explore the creation of a delocalized electron gas in the galleries (voids between planes) and show that interplane cohesion is largely due to this low-density electron gas without using Van der Waals corrections to density functional (LDA and PBE) forces. In comparison to graphene, the in-plane electronic conductivity is drastically reduced.”
Drabold, Distinguished Professor of Physics and Astronomy
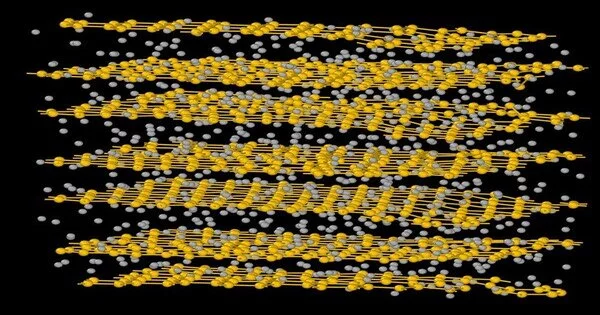
“Stomach muscle initio” signifies “all along,” and their work seeks original ways to engineer types of graphite from normally occurring carbonaceous material. What they found, with a few distinct estimations, was a layered material that structures at exceptionally high temperatures (around 3000 degrees Kelvin). Its layers stay together because of the development of an electron gas between the layers, yet they’re not the ideal layers of hexagons that make up ideal graphene. This new material has a lot of hexagons, but additionally pentagons and heptagons. That ring problem decreases the electrical conductivity of the new material contrasted with graphene, yet the conductivity is still high in the areas overwhelmed to a great extent by hexagons.
Not all hexagons
“In science, the most common way of changing carbonaceous materials over completely to a layered graphitic structure by warm treatment at high temperatures is called graphitization. In this letter, we show from stomach muscle initio and AI sub-atomic powerful reenactments that unadulterated carbon networks have a staggering proclivity to change over completely to a layered construction in a critical thickness and temperature window, with the layering happening in any event, for irregular beginning designs. “The level layers are shapeless graphene: topologically cluttered three-facilitated carbon particles organized in planes with pentagons, hexagons, and heptagons of carbon,” said Drabold, Distinguished Professor of Physics and Astronomy in the College of Arts and Sciences at Ohio University.
“Since this stage is topologically cluttered, the standard thing’stacking library’ of graphite is just genuinely regarded,” Drabold said. “The layering is seen without Van der Waals remedies to thickness useful (LDA and PBE) powers, and we examine the development of a delocalized electron gas in the displays (voids among planes) and show that interplane union is somewhat due to this low-thickness electron gas. “The in-plane electronic conductivity is emphatically decreased in comparison with graphene.”
The specialists anticipate that their declaration should spike trial and error and review tending to the presence of shapeless graphite, which might be testable from peeling or potentially exploratory surface underlying tests.
Trembly, Russ Professor of Mechanical Engineering and Director of the Institute for Sustainable Energy and the Environment in Ohio University’s Russ College of Engineering and Technology, has been working on green coal applications to a limited extent.He and Drabold — alongside physical science doctoral students Rajendra Thapa, Chinonso Ugwumadu, and Kishor Nepal — worked together on the exploration. Drabold is likewise important for the Nanoscale and Quantum Phenomena Institute at OHIO, and he has distributed a progression of papers on the hypothesis of nebulous carbon and indistinct graphene. Drabold also emphasized the outstanding work of his alumni understudies in conducting this investigation.
Surprising interplane union
“The inquiry that drove us to this is whether we could make graphite from coal,” Drabold said. This paper doesn’t completely address that inquiry, yet it shows that carbon has a mind-boggling propensity to layer — like graphite, yet with many ‘abandons’ like pentagons and heptagons (five-and seven-part rings of carbon iotas), which fit normally into the organization. We present proof that undefined graphite exists, and we portray its course of arrangement. “It has been thought from tests that graphitization happens at close to 3,000 K, yet the subtleties of the development cycle and nature of confusion in the planes were obscured,” he added.
The Ohio University specialists’ work is likewise a forecast of another period of carbon.
Until we did this, it was not the slightest bit clear that layers of undefined graphene (the planes including pentagons and heptagons) would stay together in a layered design. I see that as very amazing, and almost certainly, experimentalists will go chasing after this stuff now that its presence is anticipated, “Drabold said. “Carbon is the supernatural occurrence component—you can make life, jewels, graphite, Bucky Balls, nanotubes, graphene, then this happens. There is a great deal of intriguing fundamental material science with regards to this, as well—for instance, how and why the planes tie; this without help from anyone else is very amazing for specialized reasons.