Researchers have successfully mass-produced fat tissue in the lab that resembles the texture and composition of naturally occurring animal fats.
The findings, which were detailed in a study published today in eLife, could be used to produce cultured meat made entirely from cells, giving it a more authentic texture and flavor.
According to startup companies around the world, the development of cell-grown chicken, beef, pork, and fish has made headlines recently. These products are typically still in the early stages of development and are not yet authorized for commercial sale, nor are they ready for large-scale production, with a few exceptions.
The majority of those products are unstructured cell assemblages, like chicken nuggets as opposed to a slice of chicken breast, and are therefore still in the development stage. The texture of actual meat, which is made up of muscle fibers, connective tissue, and fat—and it is the fat that gives meat flavor—is lacking.
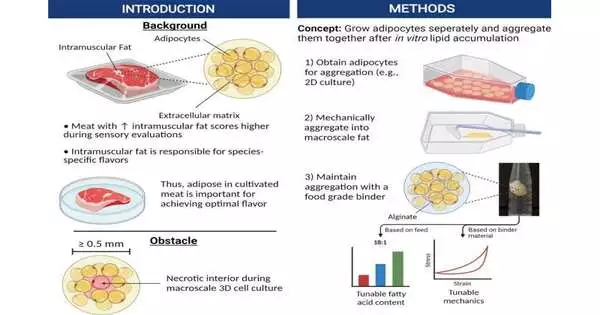
“Our objective was to create a straightforward way of manufacturing bulk fat. We reasoned that because fat tissue is mostly cells with few other structural components, aggregating the cells after development would be enough to replicate the flavor, nutrition, and texture profile of real animal fat.”
John Yuen Jr, a graduate student at the Tufts University Center for Cellular Architecture (TUCCA),
In fact, consumer testing on natural beef with varying fat contents revealed that beef with 36% fat received the highest ratings.
The cells in the middle of the fat become starved of oxygen and nutrients as the fat grows into a mass, which makes it extremely difficult to produce cultured fat tissue in sufficient quantities. Throughout the body, blood vessels and capillaries carry oxygen and nutrients. Researchers can only grow muscle or fat to a few millimeters in size because they are still unable to replicate that vascular network on a large scale in lab-grown tissue.
This restriction was overcome by the researchers by first growing fat cells from mice and pigs in a flat, two-dimensional layer. These cells were then harvested and aggregated into a three-dimensional mass using a binder like alginate or mTG, both of which are already present in some foods.
“Our aim was to produce bulk fat using a relatively straightforward process. According to first author John Yuen Jr., a graduate student at the Tufts University Center for Cellular Architecture (TUCCA), Massachusetts, US, “since fat tissue is primarily made up of cells with few other structural components, we thought that aggregating the cells after growth would be sufficient to reproduce the taste, nutrition, and texture profile of natural animal fat. Since we can gather the fat in large quantities, there is no need to maintain the cells’ viability when making tissue solely for food.”.
The aggregated fat cells immediately resembled fat tissue, but the team performed a number of additional tests to determine whether they accurately mimicked the characteristics of native fat from animals.
In order to better understand the texture, they first compressed the fat tissue to compare its resistance to pressure to that of animal fat. They discovered that cell-grown fat bound with sodium alginate could withstand pressure in a manner comparable to that of fat from animals and birds, whereas cell-grown fat bound with mTG behaved more like rendered fat, comparable to lard or tallow. This suggests it might be possible to use various types and concentrations of binders to fine-tune the texture of cultured fat so it most closely resembles the texture of fat found in meat.
Numerous flavors are released into the meat during cooking, and the majority of these flavors come from fat, particularly lipids and the fatty acids that make up these lipids. The team discovered that the mixture of fatty acids in cultured mouse fat was different from that in native mouse fat when they looked at the composition of molecules in the cell-grown fat.
The fatty acid composition of the cultured pig fat was, however, much more similar to that of the native tissue. According to the team’s preliminary research, it may be possible to add the necessary lipids to developing fat cells in order to make them more similar to the composition of natural meat.
Senior author David Kaplan, Stern Family professor of Biomedical Engineering at Tufts University and director of TUCCA, says that this approach of aggregating cultured fat cells with binding agents can be translated to large-scale production of cultured fat tissue in bioreactors—a significant barrier in the development of cultured meat.
“We continue to examine every aspect of cultured meat production with the goal of enabling the mass production of meat that resembles real meat in appearance, flavor, and texture.”.
More information: John Se Kit Yuen Jr et al, Aggregating in vitro-grown adipocytes to produce macroscale cell-cultured fat tissue with tunable lipid compositions for food applications, eLife (2023). DOI: 10.7554/eLife.82120