Each second in our bodies’ cells, endless exercises crucial to life happen thanks to proteins. These unique proteins go about as impetuses by speeding up the speed and working on the selectivity of compound responses without going through long-lasting changes themselves. Aside from their importance in science, proteins are also essential in a variety of processes in the food, drug, farming, and beauty care products industries.
Belying their universality and significance, proteins are inadequately perceived. Specifically, researchers need to understand what makes proteins’ dynamic locales—the pocket-like area where the accelerated compound responses occur—so strong. While the three-layered nuclear designs of many proteins’ dynamic locales have been pictured and planned, the “undetectable” construction of the electric fields inside a functioning site is generally obscure. These electric fields are thought to play an important role in shaping an exact climate in dynamic environments where atoms respond and rapidly change to new atoms.
A current review co-authored by Stanford scientists Chu Zheng and Yuezhi Mao has another test for estimating and imagining the electric fields inside a protein’s dynamic site. The paper, as of late distributed in the journal Nature Chemistry, describes the direction of electric fields at the site of the response and could assist analysts with computing the vital compound connections in dynamic locales. These experiences, thus, could prompt the structure of uniquely customized engineered proteins for industry, as well as enormously propel the revelation and planning of new medications that impede or tweak the capability of chemical targets.
“We have developed a novel probe that can provide us with important information about how electric fields are uniquely oriented in enzymes, which we believe is fundamental to enzymes’ amazing catalytic power,”
Zheng, a graduate student in the lab of Steven G. Boxer
“We have fostered a clever test that can give us significant data about how electric fields are remarkably situated in proteins, which we believe is key to the astounding reactant force of catalysts,” said Zheng, an alumni understudy in the lab of Steven G. Fighter, the Camille Dreyfus Professor of Chemistry.
At an essential level, we are attempting to more readily comprehend how proteins work, and in this review, we are adding another aspect by getting electric field directions, which is accepted to basically affect a catalyst’s reactant capabilities,” said Mao, a postdoctoral researcher in science who works in the lab of Thomas Markland, an academic partner in science at Stanford and, furthermore, a senior co-writer.
A strong new device
The Boxer lab at Stanford has spearheaded the idea of deciphering the usefulness of proteins by estimating electrostatic connections, which are available in all types of issues and are explicitly coordinated in three aspects in huge natural particles.
“The beginning of the astounding usefulness of proteins is a general inquiry, and it applies to organic catalysis as well as compound catalysis too—which is an immense business,” Boxer said. “About 80% of all synthetics are made utilizing impetuses, yet what is really liable for bringing down the enactment free energy [to cause the response to happen faster] isn’t known for most responses. Examining the role of electric fields in protein capability is especially at the core of our work, “said Boxer, who is the chair of the Department of Chemistry at Stanford’s School of Humanities and Sciences and a senior co-creator of the review.
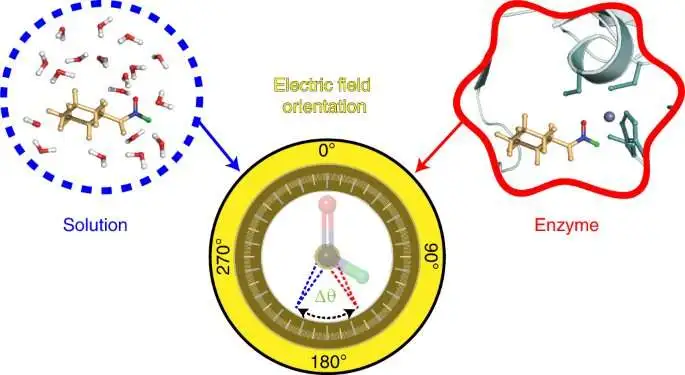
The test created by the Stanford group depends on a method — likewise created in the Boxer lab — called vibrational Stark impact spectroscopy. This method estimates the vibrational frequencies in test atoms in view of the frequency of infrared light consumed by their compound bonds. Changes in these vibrational frequencies uncover data about the electric fields present. In this review, the scientists explored shifts in the vibrational frequencies of compound bonds in a test produced using a particle called N-cyclohexylformamide. This atom goes about as an inhibitor, restricting to the dynamic site of a protein called liver liquor dehydrogenase.
To picture the electric field in the dynamic site of liver liquor dehydrogenase, the analysts designated two bonds in the N-cyclohexylformamide test at around 120 degrees from one another. That particular point between the two bonds permitted the analysts to check not just the strength, or size, of the electric field, but in addition the field’s direction. Past examinations from the Boxer lab on other protein dynamic locales had covered the size of electric fields but not on their headings.
“We call this device a two-directional test on the grounds that with this test we can gauge the electric field in a functioning site in two unique bearings,” Zheng said. “Utilizing the test along these lines, we can remake and concentrate the direction data about the electric field.” That hasn’t been finished before. “
Gathering this key estimate initially required some compound skillful deception. One of the N-cyclohexylformamide test’s compound bonds—between a carbon iota and a hydrogen molecule—is famously hard to see in protein conditions. Thus, the analysts traded the hydrogen iota for the component’s heavier cousin, called deuterium. The new carbon-deuterium bond demonstrated amiable to estimation and assisted the analysts with uncovering the direction of the electric field.
A precise enzymatic environment
The Stanford analysts joined their trial information with virtual experiences and quantum mechanical estimations to depict the electric field’s connections with N-cyclohexyl formamide, altered with deuterium, at the dynamic site of liver liquor dehydrogenase. Those properties were then contrasted with the electric fields tracked down in water, CH3CO, and other normal solvents.
Quite, the analysts found the direction of the electric field in the dynamic site of liver liquor dehydrogenase varied extensively from the electric field direction in the solvents they examined. That outcome upholds the possibility that protein dynamic locales highlight what researchers call a preorganized electrostatic climate, or one in which the exact situating of amino acids and the electrostatic climate they create assist in lessening the energy expected for a compound response to occur. This could be a key to proteins’ striking ability to catalyze responses.
“With this review, we are assisting in propelling the idea of relating the exhibition of proteins with both the size and direction of the electric fields in dynamic locales,” Mao said. “What we have found is proof that electric fields in the protein dynamic locales are preorganized, and that is a significant hint in tackling the secret of why catalysts have their astounding skills.”
The test created by the Stanford analysts could be utilized to explore numerous other proteins’ dynamic locales. Spreading information in this manner will bring researchers and architects closer to having the option to plan custom proteins with awesome new qualities.
“A definitive objective of this exploration is to empower us to plan proteins that have great reactive execution for biomedical and modern applications,” Zheng said. “We are still far from that, yet we are gaining ground and have a preferred view currently over prior to that in regards to how proteins work.”
More information: Chu Zheng et al, A two-directional vibrational probe reveals different electric field orientations in solution and an enzyme active site, Nature Chemistry (2022). DOI: 10.1038/s41557-022-00937-w
Journal information: Nature Chemistry