Although there is currently no treatment for heart failure, the condition is prevalent. Additionally, treatments can only alleviate symptoms and frequently cause side effects. However, focusing on cardiac fibrosis—a condition that frequently precedes heart failure—in recent research that was published in ACS Biomaterials Science & Engineering may open up new avenues toward potential therapies. A more accurate model of this “scarred” cardiac tissue has been created by researchers, which may facilitate faster drug testing.
Heart failure affects millions of people when the heart is unable to pump enough blood to support the body. Outrageous cases can require medical procedures or even entire heart transfers, and these techniques can prompt entanglements.
Cardiac fibrosis, a condition in which stiff scar tissue forms around the heart and prevents it from beating properly, is one of the underlying causes of heart failure. Although there are treatments for other aspects of heart failure, cardiac fibrosis has received little attention as a potential target for new treatments.
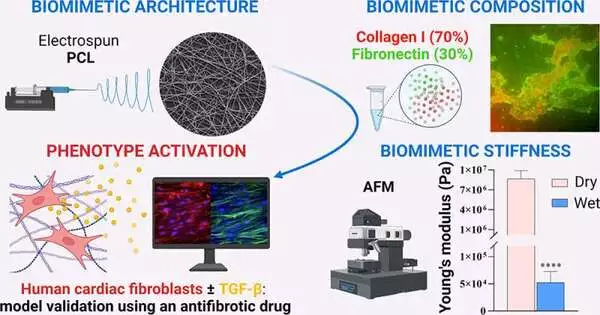
Consequently, there are not many testing platforms that replicate the condition’s unique characteristics. Animal cells, which lack some of the characteristics of human cells, or hydrogels that do not match the architecture of scarred cardiac tissue have been used in previous platforms. Therefore, Gerardina Ruocco, Irene Carmagnola, Valeria Chiono, and others wanted to create a more accurate in vitro model of human cardiac fibrotic tissue that could improve the efficiency and effectiveness of subsequent preclinical studies.
Using electrospinning, the researchers constructed a scaffold from a biodegradable polymer and a network of randomly oriented nanofibers to accomplish this. The scaffold was submerged in a solution containing collagen and fibronectin, both of which are essential components of the cardiac environment and have the potential to promote fibrosis.
Human cardiovascular cells, which structure solid scar tissue when they separate into myofibroblasts, were permitted to develop on the framework. The new model’s rigid scaffolding caused this differentiation naturally, whereas previous tissue models required specific growth factors to do so.
The team tested the model’s response to an antifibrotic drug in order to validate it. Myofibroblasts did not develop following administration, and the tissue remained flexible. According to the researchers, this model may make it possible to test new treatments for fibrosis without using animals for better accuracy.
More information: Gerardina Ruocco et al, Biomimetic Electrospun Scaffold-Based In Vitro Model Resembling the Hallmarks of Human Myocardial Fibrotic Tissue, ACS Biomaterials Science & Engineering (2023). DOI: 10.1021/acsbiomaterials.3c00483