The Ocean Side Young Men’s notable hit single “Great Vibrations” takes on an entirely different layer of importance because of a new revelation by Rice College researchers and teammates, who have revealed a method for obliterating malignant growth cells by utilizing the capacity of certain particles to vibrate firmly when invigorated by light.
When stimulated by near-infrared light, the atoms of a small dye molecule used for medical imaging can vibrate in unison—known as a plasmon—rupturing the cell membrane of cancerous cells, as the researchers discovered. The method was found to be effective against lab cultures of human melanoma cells by 99% in the Nature Chemistry study, and treatment resulted in the cancer-free recovery of half of the mice with melanoma tumors.
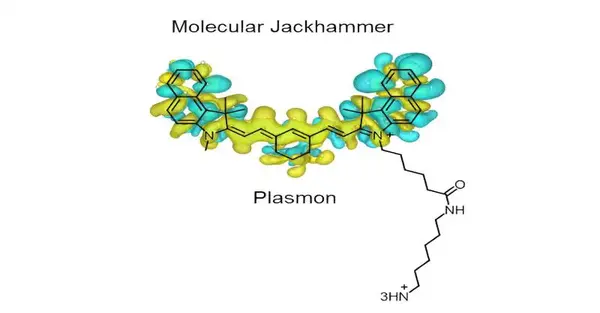
“It is a whole new generation of molecular machines that we call molecular jackhammers,” Rice chemist James Tour said. His lab has previously used nanoscale compounds with a light-activated paddle-like chain of atoms that spins in the same direction to drill through the outer membrane of infectious bacteria, cancer cells, and treatment-resistant fungi.
Dissimilar to the nanoscale drills in view of Nobel laureate Bernard Feringa’s sub-atomic engines, atomic drills utilize an altogether unique—and remarkable—system of activity.
Tour stated, “They can be activated with near-infrared light rather than visible light.” Additionally, “they are more than one million times faster in their mechanical motion than the former Feringa-type motors.”
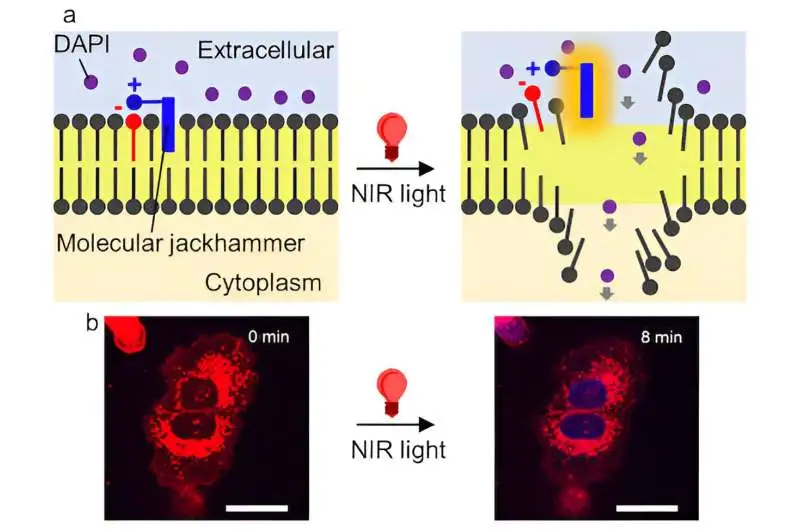
(a) A sub-atomic drill (blue) connects itself to a malignant growth cell’s lipid bilayer lining. It vibrates strongly when stimulated by near-infrared light, breaking the cell membrane. b) DAPI entering and staining the core of the layer upsets A375 melanoma cells, as pictured by fluorescence confocal microscopy. Scale bar = 25 µm. Credit: Ciceron Ayala-Orozco/Rice College
Close infrared light can infiltrate far more deeply into the body than apparent light, getting to organs or bones without harming tissue.
“Close infrared light can dive as deep as 10 centimeters (~ 4 inches) into the human body instead of just a portion of a centimeter (~ 0.2 inches), the profundity of infiltration for noticeable light, which we used to enact the nanodrills,” said Visit, Rice’s T. T.; furthermore, W. F. Chao, a teacher of science and a teacher of materials science and nanoengineering. “It is an immense development.”
The drills are aminocyanine particles, a class of fluorescently engineered colors utilized for clinical imaging.
“These particles are straightforward colors that individuals have been utilizing for quite a while,” said Ciceron Ayala-Orozco, a Rice research researcher who is the lead writer on the review. “They’re biocompatible, stable in water, and truly adept at connecting themselves to the greasy external coating of cells. Be that as it may, despite the fact that they were being utilized for imaging, individuals didn’t have the foggiest idea how to initiate these as plasmons.”
Ayala-Orozco at first considered plasmons as a doctoral understudy in the exploration bunch led by Rice’s Naomi Halas.

Ciceron Ayala-Orozco is an examination researcher in the Visit Lab at Rice College and the lead creator of the review. Credit: Jeff Fitlow/Rice College
“Because of their design and synthetic properties, the cores of these atoms can waver in a state of harmony when presented to the right upgrade,” Ayala-Orozco said. “I saw a need to utilize the properties of plasmons as a type of treatment and was keen on Dr. Visit’s mechanical way to deal with managing disease cells. I essentially came to an obvious conclusion.
“The sub-atomic plasmons we recognized have a close, balanced structure with an arm on one side. The arm doesn’t add to the plasmonic movement, yet it helps anchor the atom to the lipid bilayer of the cell film.”
The scientists needed to demonstrate that the particles’ method of activity couldn’t be sorted either as a type of photodynamic or photothermal treatment.
“What should be featured is that we’ve found one more clarification for how these atoms can function,” Ayala-Orozco said. “This is the first time that a molecular plasmon has been used in this way to excite the entire molecule and actually produce mechanical action that is used to achieve a specific goal, which is to tear the membrane off of cancer cells. This study is about an alternate method for treating disease utilizing mechanical powers at the sub-atomic scale.”
Specialists at Texas A&M College, led by Jorge Seminario, a quantum scientist and teacher of substance design, performed a time-subordinate thickness useful hypothesis examination on the sub-atomic highlights engaged with the drilling impact. The malignant growth studies were performed in mice at the College of Texas MD Anderson Disease Center as a team with Dr. Jeffrey Myers, teacher and chair of the Branch of Head and Neck. A medical procedure and overseer of translational examinations for the Division of Medical Procedure.
More information: Ciceron Ayala-Orozco et al, Molecular jackhammers eradicate cancer cells by vibronic-driven action, Nature Chemistry (2023). DOI: 10.1038/s41557-023-01383-y