Proficient adsorbents for modern wastewater treatment are essential to limit expected ecological harm. Specifically, natural colors, as a critical gathering of modern poisons, are normally profoundly water soluble, non-degradable, and many are poisonous and cancer-causing. Changxia Li and Freddy Kleitz from the Faculty of Chemistry of the University of Vienna, along with partners, have currently introduced another way to deal with plan a creative composite material, comprising of a nanoporous, ultrathin covalent natural structure (COF) secured on graphene, that is exceptionally effective at separating natural contamination from water. The review was published in Angewandte Chemie.
“There are multiple ways, including enacted carbon channels, to filter water today, yet there is still an opportunity to get better in the effectiveness or adsorption limit of the applications,” says first creator and postdoctoral specialist Changxia Li.
“Using water, we created a unique process for forming COF that is rather environmentally benign. As a result, we were able to create small ‘sponges’ with nanometer-sized pore sizes and shapes, as well as a tuned negative surface charge that was very selective in pulling the positively charged target molecules, i.e., our dyes, out of the water.”
Changxia Li.
Freddy Kleitz’s gathering at the Institute of Inorganic Chemistry—Functional Materials is creating novel nanoporous materials. Permeable materials have a much bigger complete surface region than non-permeable materials for a similar volume and can, in this manner, gather an especially huge number of particles on their surfaces throughout adsorption.
Profoundly permeable COF as another class of material
Covalent natural systems (COFs) are a moderately clever class of materials. They are especially permeable while simultaneously being low-thickness and lightweight. Covalent implies that their synthetic bonds are framed by means of electron matches between particles.
The colors the analysts concentrated on in their watery model arrangement were around 0.8 to 1.6 nanometers in size. “We fostered an original strategy to frame COF in a similarly harmless way to the ecosystem, utilizing water.” Thusly, we had the option to foster little ‘wipes’, with planned pore sizes and pore shapes in the nanometer range, as well as a tuned negative surface charge, that was exceptionally particular in pulling the decidedly charged target particles, i.e., our colors, out of the water. “Very much like the wipe absorbs the water, just for our situation it’s the contamination.”
A spine made from graphene
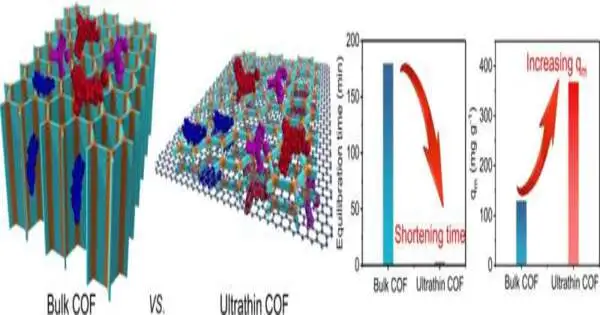
While utilizing mass COF powder, the internal pores of the material are many times at this point not open to contamination because of pore blockage at the external edge, particularly for enormous poison particles. The original composite material created by the specialists offers a completely porous design: For this reason, the scientists developed COF on flimsy layered graphene nanosheets. The mix of graphene—in itself currently a 2D layer of carbon particles—and the layer of COF, which is only two nanometers thick, brought about a smaller, open 3D design. The ultrathin COF layer could uncover more adsorption locales than the mass of COF powder.
Then again, the bigger, honeycomb-like pores of the graphene network support the vehicle of water through the channel material. The huge pores of the graphene network in combination with the ultrathin COF layer provide an enormous measure of adsorption destinations, hence empowering especially quick as well as effective wastewater treatment, the scientists said. Because of the nearly low material contribution of graphene as well as the likelihood of reusing the composite material — after the poisons have been cleaned out — as a channel, the improvement is likewise somewhat cost-effective, they said.
More information: Changxia Li et al, Ultrathin Covalent Organic Framework Anchored on Graphene for Enhanced Organic Pollutant Removal, Angewandte Chemie International Edition (2022). DOI: 10.1002/anie.202206564
Journal information: Angewandte Chemie International Edition , Angewandte Chemie