Researchers from Stanford College and the Branch of Energy’s Oak Edge Public Lab are transforming air into manure without leaving a carbon impression. Their disclosure could convey a truly necessary answer to assist with meeting overall carbon-nonpartisan objectives by 2050.
Distributed in the journal Energy and Ecological Science, the review portrays a practical electrochemical—as opposed to compound—process for creating smelling salts, a critical element for nitrogen manure.
Fundamentally, the specialists utilized neutron dispersing to comprehend how cycling an electric flow during the change of nitrogen to alkali, otherwise called the nitrogen decrease response, expands how much smelling salts are created. This cycle can possibly empower ranchers to change nitrogen, the most abundant component in our environment, into salt-based manures without producing carbon dioxide.
“Ammonia is critical to food supplies for the majority of the world’s population. As the world’s population continues to grow, we need more sustainable ways to produce fertilizers, especially as global warming intensifies.”
Sarah Blair, a former doctoral student at Stanford’s Center for Interface Science and Catalysis.
“Smelling salts is basic to food supplies for a large portion of the total populace,” said Sarah Blair, a previous doctoral understudy at Stanford’s Middle for Connection Point Science and Catalysis who presently works at the Public Sustainable Power Lab in Colorado as a postdoctoral scientist. “As the total populace keeps on developing, we really want practical ways of delivering compost, particularly as warming increases.”
Modern composts permit ranchers to develop more food on less land. However, the essential strategy to make modern-smelling salts for over a hundred years, the Haber-Bosch process, represents almost 2% of all carbon dioxide outflows due to the non-renewable energy sources it requires.
Two percent probably won’t seem like a great deal, yet we are adding carbon dioxide to the climate quicker than the planet can ingest it, making the most of a significant investment of time and energy toward lessening that number. The Haber-Bosch process produces around 500 million tons of carbon dioxide every year, which would require what could be compared to practically each of the government lands in the U.S. to ingest and store.
Experiences from the review could likewise assist researchers with figuring out different cycles for making carbon-unbiased alkali for different applications. These could incorporate reusing or recovering manure overflow before it enters water streams and creating smelling salts at seaports for energizing boats. Worldwide transportation creates another 3% of the world’s carbon dioxide outflows, and petroleum product burning records the biggest wellspring of carbon dioxide from human movement.

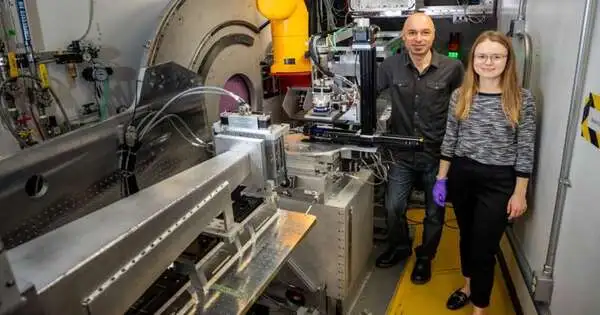
Blair utilized a glovebox in the tests, which required close joint effort and a cautious plan by Doucet so the venture could capitalize on restricted shaft time. Credit: Genevieve Martin/ORNL, U.S. Dept. of Energy
“You can’t work on the plan of something on the off chance that you don’t have any idea how it’s as of now functioning,” Blair said. “Neutrons assist science with advancing by revealing insight at the nuclear level on specific frameworks that are difficult to concentrate on in any case.”

Blair utilized a glovebox in the tests, which required close cooperation and a cautious plan by Doucet so the task could capitalize on restricted pillar time. Credit: Genevieve Martin/ORNL, U.S. Dept. of Energy
Blair and Mat Doucet, a senior neutron dispersing researcher at ORNL, directed their neutron probes at the Fluids Reflectometer instrument at the Spallation Neutron Source. Their point was to figure out the impact of cycling an electric flow on the development of the strong electrolyte interface, or SEI, in a nitrogen decrease response framework that produces smelling salts involving lithium as a middle person.
Understanding SEI development holds the key, not exclusively to opening up the science behind the electrochemical creation of alkali, but in addition to delivering better batteries. The concentrate additionally denotes the primary utilization of neutron-based strategies to notice the arrangement of a SEI layer during this specific electrochemical change.
Likewise, a solitary new neutron method, time-settled reflectometry, rose out of the review. This procedure permits researchers to cut neutron information into additions of a couple of moments, catching more significant subtleties, similar to watching a film outline by outline. At first, Blair and Doucet thought the electrochemical changes they noticed happened continuously. Nonetheless, because of the new method, they found changes occurring in a much more modest amount of time.
“Processes that seem, by all accounts, to be direct probably won’t be straight at all when you take a gander at them all the more intently,” Doucet said. “Getting to that design as an element of time is the crucial step. The strategy we produced for this examination permitted us to do exactly that.”
Blair, previously of Stanford, utilized the Fluids Reflectometer at Oak Edge Public Lab in her doctoral examination. Credit: Genevieve Martin/ORNL, U.S. Dept. of Energy
Revelations on SNS establish the underpinnings of information for mechanical advancements that work on individuals’ day-to-day routines. The procedure Blair and Doucet created opens additional opportunities in electrochemistry for SNS clients.
Hanyu Wang, an ORNL instrument researcher who additionally works intimately with SNS clients, said, “These time-subordinate investigations will draw researchers who concentrate on division sciences.”
ORNL Neutron Reflectometry bunch pioneer Jim Cooking added, “Their methodology can respond to a great deal of inquiries for division sciences, batteries, and a whole range of various areas of interest, similar to energy creation, energy capacity, and preservation of energy.”
More information: Sarah J. Blair et al. Combined, time-resolved, in situ neutron reflectometry and X-ray diffraction analysis of dynamic SEI formation during electrochemical N2 reduction, Energy & Environmental Science (2023). DOI: 10.1039/D2EE03694K