Epilepsy is a neurological problem that arises from strange electrical movements in the cerebrum, prompting seizures. These seizures can be caused by a variety of factors, including hereditary variations in a group of proteins that regulate potassium particles in the brain.Scientists at Washington University in St. Louis have led a worldwide group to investigate the instruments behind the capability and brokenness of these proteins, as well as their communication with an antiepileptic drug, to foster a possible new system to treat epilepsy.
Jianmin Cui, teacher of biomedical design in the McKelvey School of Engineering, and Nien-Du Yang, a doctoral understudy in biomedical design who conducts research in Cui’s lab, collaborated with Harley Kurata, academic partner of pharmacology at the University of Alberta, and examined the functioning component of two potassium particle channels, KCNQ2 and KCNQ3. Their discoveries reveal a rationed instrument for KCNQ channel initiation that is an objective of both epilepsy-connected changes and a small particle compound.
The work was published July 20 in Science Advances.
The KCNQ potassium channel family has a variety of functions, ranging from directing heartbeat (via KCNQ1) to controlling neuron volatility (via KCNQ2-5).These channels are voltage-actuated, so they sense voltage changes across the cell layer and open and close accordingly. The correspondence between voltage detecting and channel pore opening is known as electro-mechanical coupling, an interaction including conformational changes of the protein during voltage-subordinate enactment.
“It’s unclear if the neuronal KCNQ isoforms share the same electro-mechanical coupling mechanism or two open states despite the fact that KCNQ channels have remarkably comparable sequences and shapes. This research highlights fundamental parallels and divergences between these channels that could have significant effects on how well they function in cardiomyocytes or neurons.”
Nien-Du Yang, a doctoral student in biomedical engineering
Cui’s group has recently shown that KCNQ1, the cardiovascular KCNQ isoform, highlights a two-stage process in electro-mechanical coupling that prompts two unmistakable channel open expresses, the moderate open and the enacted open. KCNQ1’s tissue-explicit tweaks, infection pathogenesis and pharmacology underlie KCNQ1’s tissue-explicit tweaks. KCNQ2 and KCNQ3 are exceptionally well communicated in the focal sensory system and are the primary supporters of the M-current, a basic potassium current that balances neuronal volatility. Thusly, hindered M-current capability by innate transformations in KCNQ2 and KCNQ3 is ordinarily connected with early-stage and pediatric epilepsy.
“In spite of the fact that KCNQ diverts are profoundly comparable in their successions and designs, whether the neuronal KCNQ isoforms additionally share the equivalent electro-mechanical coupling component or two open states is hazy,” said Yang, the paper’s most memorable creator. “This work uncovers key likenesses and contrasts between these channels that might have significant ramifications for their capability in cardiomyocytes or neurons.”
The group utilized different techniques to study the electro-mechanical coupling system in these potassium channels, including making explicit hereditary transformations for the channels, electrophysiology, and fluorescence optical estimations.
“Explaining the atomic system for electro-mechanical coupling is a significant stage toward understanding the voltage-subordinate gating of potassium channels,” Cui said. “We gave useful proof that the neuronal KCNQ2 and KCNQ3 channels are not the same as KCNQ1 in that they highlight a solitary enacted open state yet with a monitored electro-mechanical coupling system explicit for the initiated open state.”
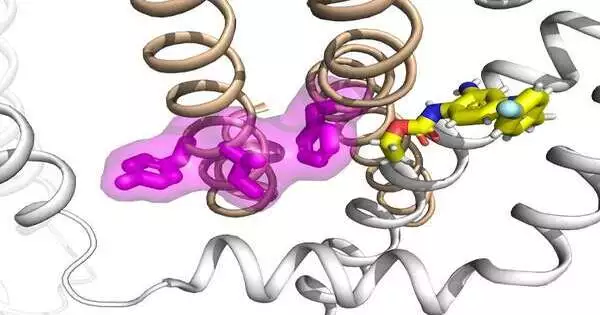
The specialists found that these channels are practical objectives for medicines for epilepsy. The group likewise recognized a bunch of changes in KCNQ2 and KCNQ3 related to early childhood epilepsy encephalopathy, a serious type of young life epilepsy that explicitly disturbs the electro-mechanical coupling of the channels. The scientists exploited an antiepileptic model medication, retigabine, given its instrument of activity on neuronal KCNQ channels, and exhibited that the electro-mechanical coupling can be straightforwardly improved to protect the capability of these sick freaks. Their examinations recommend that the electro-mechanical coupling system in KCNQ channels can be a compelling objective, introducing an original pharmacological procedure for developing more viable treatments for epilepsy treatment.
More information: Nien-Du Yang et al, Electro-mechanical coupling of KCNQ channels is a target of epilepsy-associated mutations and retigabine, Science Advances (2022). DOI: 10.1126/sciadv.abo3625
Journal information: Science Advances