Specialists led by Teacher Kang Kisuk of the Middle for Nanoparticle Exploration inside the Establishment for Fundamental Science (IBS) have reported a significant forward leap in the field of cutting-edge, strong-state batteries. It is accepted that their new discoveries will empower the making of batteries in view of a clever chloride-based strong electrolyte that displays remarkable ionic conductivity.
A squeezing worry with current business batteries is their dependence on fluid electrolytes, which prompts combustibility and blast chances. Consequently, the advancement of non-flammable, strong electrolytes is of vital significance for propelling strong-state battery innovation.
As the world pinion wheels up to manage gas-powered motor vehicles and grow the utilization of electric vehicles in the continuous worldwide shift toward feasible transportation, examination into the center parts of auxiliary batteries, especially strong state batteries, has picked up huge speed.
“This newly discovered chloride-based solid electrolyte has the potential to overcome the limitations of traditional sulfide and oxide-based solid electrolytes, bringing us one step closer to the widespread adoption of solid-state batteries.”
Corresponding author Kang Kisuk,
To make strong-state batteries pragmatic for ordinary use, it is essential to foster materials with high ionic conductivity, vigorous synthetic and electrochemical security, and mechanical adaptability. While past exploration effectively prompted sulfide and oxide-based strong electrolytes with high ionic conductivity, none of these materials completely met this multitude of fundamental prerequisites.
Previously, researchers have likewise investigated chloride-based strong electrolytes, known for their unrivaled ionic conductivity, mechanical adaptability, and security at high voltages. These properties led some to guess that chloride-based batteries are the most probable possibility for strong-state batteries. Notwithstanding, these expectations rapidly vanished, as the chloride batteries were viewed as unfeasible because of their weighty dependence on costly interesting earth metals, including yttrium, scandium, and lanthanide components, as auxiliary parts.
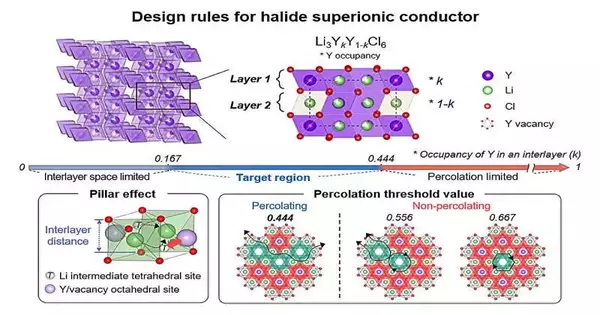
To address these worries, the IBS research group checked out the dissemination of metal particles in chloride electrolytes. They accepted the explanation that three-sided chloride electrolytes can achieve low ionic conductivity depending on the variety of metal particle plans inside the design.
They initially tried this hypothesis on lithium yttrium chloride, a typical lithium metal chloride compound. At the point when the metal particles were situated close to the pathway of lithium particles, electrostatic powers caused obstacles in their development. On the other hand, assuming the metal particle inhabitance was too low, the way for lithium particles turned out to be too restricted, blocking their versatility.
Expanding on these experiences, the exploration group acquainted systems with plan electrolytes in a manner that mitigates these clashing variables, at last prompting the effective improvement of a strong electrolyte with high ionic conductivity. The gathering went further to effectively show this system by making a lithium-metal-chloride strong-state battery in view of zirconium, which is far less expensive than the variations that utilize uncommon earth metals.
Here, the meaning of the metal particle game plan on a material’s ionic conductivity was illustrated.
This exploration uncovers the frequently neglected job of metal particle circulation in the ionic conductivity of chloride-based strong electrolytes. It is normal that the IBS Center’s exploration will prepare for the advancement of different chloride-based strong electrolytes and further drive the commercialization of strong state batteries, promising better moderateness and security in energy capacity.
Comparing writer Kang Kisuk states, “This newfound chloride-based strong electrolyte is ready to rise above the impediments of regular sulfide and oxide-based strong electrolytes, carrying us one bit nearer to the far and wide reception of strong state batteries.”
The paper is distributed in the journal Science.
More information: Seungju Yu et al, Design of a trigonal halide superionic conductor by regulating cation order-disorder, Science (2023). DOI: 10.1126/science.adg6591. www.science.org/doi/10.1126/science.adg6591